Scientists can calculate the mass of the ions using this kinetic energy and their time taken to travel a fixed distance down the flight tube. Recent innovations have expanded MS to clinical and single-cell proteomics. A sequence of just a few amino acids and the flanking masses a peptide sequence tag is sufficient for identifying a peptide from the entirety of human proteome. In MS we ionize a sample by essentially firing electrons at the sample which has the effect of knocking other electrons bound to the sample particles off. A precise assignment could be made from a high-resolution m/z value (next section). TOF spectrometry may sound like a complicated process, but it has just four simple stages: Lets explore those steps in more detail.
Recent efforts extend this functionality by incorporating standard or proteomic-specific bioinformatics pipelines, including machine learning, and by integrating the proteomic data with other omics-type data such as various flavours of next-generation sequencing (NGS).
The molecules of these compounds are similar in size, CO2 and C3H8 both have a nominal mass of 44 Da, and C3H6 has a mass of 42 Da. This is to prevent the ions colliding with air particles. Some common applications of MS-based proteomics in biology. Otherwise, why would the charge be predictable? Furthermore, there are many more protein copies compared to their corresponding mRNAs, making single-cell proteomics inherently more robust. Liquid chromatography (LC) is a technique widely used to separate compounds from a sample prior to analysis and is frequently coupled to mass spectrometry. If the ions are all isotopes of the same element, we can then work out relative atomic mass. Now one question that you might
Set individual study goals and earn points reaching them. A few such mechanisms are shown above. The sample preparation ends with hundreds of thousands of purified peptides produced from tens of thousands of proteins, with a million-fold concentration differences or more. This influence is thought to occur because of a "localization" of the radical cation component of the molecular ion on the heteroatom. The ions are then detected electronically and the resulting information is stored and analyzed in a computer. Rev. As the Larmor frequency is dependent upon the intensity of the magnetic field, it varies from instrument to instrument. The technique is useful for identifying and quantifying the Fundamentally, all ions are separated by modulating their trajectories in electrical fields. Precursor ions with a specific m/z are first isolated by the quadrupole and fragmented through collision with inert gases such as N2, He or Ar. Chem. unified atomic mass units. have been asking yourself is how have chemists been able to figure out what the various isotopes
Menu Close Consequently, the radical cation character of the molecular ion (m/z = 170) is delocalized over all the covalent bonds.
The masses of molecular and fragment ions also reflect the electron count, depending on the number of nitrogen atoms in the species. It can be used to measure relative isotopic concentration, atomic and molecular mass, and the compound structure. A mass spectrum will usually be presented as a vertical bar graph, in which each bar represents an ion having a specific mass-to-charge ratio (m/z) and the length of the bar indicates the relative abundance of the ion. Flight tubes? The proteome is the collection of proteins present in biofluids, cells and tissues and reflects the functional state of the biological system. A major caveat of the typically used TMT (tandem mass tag) isobaric labelling method is that co-fragmented peptides can suppress quantitative differences (ratio compression). All mass spectrometers have three fundamental components: an ion source, mass analyser and detector (Figure 1A). or an even # N atoms, odd-electron ions Since there are no heteroatoms in this molecule, there are no non-bonding valence shell electrons. Anal. bunkers for sale in california. As all ions are accelerated to the same kinetic energy, lighter ions have a faster velocity. Because the ions should all have a charge of +1, the mass-to-charge ratio simply represents their mass. Mass analysers differ in the principle they use for separating ions, and this defines their preferred application areas. A sample of neon gives the following data. and Kirkpatrick, D.S. { Mass_spectrometry_1 : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
In a spectrometer, tin-49 ions are accelerated to J. odd-number mass, odd-electron ions So for this sample: Sometimes you may not be asked to find the abundance of ions or molecules, but instead information such as their velocity. And it all comes from this has a mass number of 96, you have a little bit more versions isotopes. Charting the extent, nature and temporal progression of PTMs has arguably been the most substantial contribution of MS-based proteomics to biology.
And the ions that have a Modern mass spectrometers easily distinguish (resolve) ions differing by only a single atomic mass unit, and thus provide completely accurate values for the molecular mass of a compound. Genes are the unit of heredity, but they only come to life when they are translated to proteins the primary functional actors in biology. He is a director at the Max Planck Institute of Biochemistry, Munich and also director of the Department of Proteomics, Novo Nordisk Foundation Center for Protein Research at the University of Copenhagen.
 An animated display of this ionization process will appear if you click on the ion source of the mass spectrometer diagram. How to read an NMR spectrum and what it tells you The signal detected by an NMR spectrometer (the FID) must be transformed prior to analysis. However, this selection is partly stochastic as there are more peptides than analysis time, and it generates missing values. The grey arrow indicates changes in protein abundances. This post will walk you through the steps to fully characterize a molecule by 1- and 2-dimensional NMR, including on how to perform NMR interpretation.
An animated display of this ionization process will appear if you click on the ion source of the mass spectrometer diagram. How to read an NMR spectrum and what it tells you The signal detected by an NMR spectrometer (the FID) must be transformed prior to analysis. However, this selection is partly stochastic as there are more peptides than analysis time, and it generates missing values. The grey arrow indicates changes in protein abundances. This post will walk you through the steps to fully characterize a molecule by 1- and 2-dimensional NMR, including on how to perform NMR interpretation. To this end, extracted and solubilized proteins are digested with a sequence-specific protease. as mass spectrometry. Nie wieder prokastinieren mit unseren Lernerinnerungen.
 You may know the equation linking energy (KE), velocity (v) and mass (m): We can rearrange this to make velocity the subject. The weak even -electron ions at m/z=15 and 29 are due to methyl and ethyl cations (no nitrogen atoms). The phase of the sample could be a solid or a liquid too. Phosphorylation is the most studied PTM, and titanium dioxidebased beads are frequently used for enriching phosphopeptides with a high specificity. Rev. Nature537, 347355, 10.1038/nature19949, Geyer, P.E., Holdt, L.M., Teupser, D. and Mann, M. (2017) Revisiting biomarker discovery by plasma proteomics. Direct link to Cara Goodman's post How do you determine the , Posted 2 years ago. the charge of the ions. We know that the abundance of gaseous peptide ions is proportional to their original concentration, making it beneficial to use the lowest flow rates possible, thereby maximizing sensitivity. atomic mass units. Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. Be perfectly prepared on time with an individual plan. Often, proteins are isolated after a biochemical enrichment procedure appropriate to the question at hand, such as cellular fractionation, affinity enrichment or proximity assays. Drug repurposing or repositioning (DR) refers to finding new therapeutic applications for existing drugs. Direct link to CavCave's post This is probably way too , Posted 2 years ago. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. This tutorial mainly focuses on the interpretation of mass spectra containing multiply charged molecules generated by the electrospray ionization process. a bunch of electrons. When a single electron is removed from a molecule to give an ion, the total electron count becomes an odd number, and we refer to such ions as radical cations. These are accompanied by a set of corresponding alkenyl carbocations (e.g. Quadrupoles, usually combined with time-of-flight (TOF) or Orbitrap analysers, are the most common in proteomics.
You may know the equation linking energy (KE), velocity (v) and mass (m): We can rearrange this to make velocity the subject. The weak even -electron ions at m/z=15 and 29 are due to methyl and ethyl cations (no nitrogen atoms). The phase of the sample could be a solid or a liquid too. Phosphorylation is the most studied PTM, and titanium dioxidebased beads are frequently used for enriching phosphopeptides with a high specificity. Rev. Nature537, 347355, 10.1038/nature19949, Geyer, P.E., Holdt, L.M., Teupser, D. and Mann, M. (2017) Revisiting biomarker discovery by plasma proteomics. Direct link to Cara Goodman's post How do you determine the , Posted 2 years ago. the charge of the ions. We know that the abundance of gaseous peptide ions is proportional to their original concentration, making it beneficial to use the lowest flow rates possible, thereby maximizing sensitivity. atomic mass units. Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. Be perfectly prepared on time with an individual plan. Often, proteins are isolated after a biochemical enrichment procedure appropriate to the question at hand, such as cellular fractionation, affinity enrichment or proximity assays. Drug repurposing or repositioning (DR) refers to finding new therapeutic applications for existing drugs. Direct link to CavCave's post This is probably way too , Posted 2 years ago. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. This tutorial mainly focuses on the interpretation of mass spectra containing multiply charged molecules generated by the electrospray ionization process. a bunch of electrons. When a single electron is removed from a molecule to give an ion, the total electron count becomes an odd number, and we refer to such ions as radical cations. These are accompanied by a set of corresponding alkenyl carbocations (e.g. Quadrupoles, usually combined with time-of-flight (TOF) or Orbitrap analysers, are the most common in proteomics. deflected different amounts as they go through the magnetic field. Mol. He has obtained numerous prizes and is one of the most cited scientists with an h-factor of 232 and 250,000 total citations. of the users don't pass the Mass Spectrometry quiz! If your ions have a different charge, well, then you would have to make the appropriate adjustment. The separated ions are then measured, and the results displayed on a chart. If the former, then how can we make inferences of the abundance of isotopes in nature from a single sample? The chromatographic retention time is an important level of information when matching a dataset against a previous measurement and is key to targeted proteomics technologies. A perpendicular magnetic field deflects the ion beam in an arc whose radius is inversely proportional to the mass of each ion. Biol. Even though extensive fragmentation has occurred, many of the more abundant ions (identified by magenta numbers) can be rationalized by the three mechanisms shown above. Residual energy from the collision may cause the molecular ion to fragment into neutral pieces (colored green) and smaller fragment ions (colored pink and orange). 66, 43904399, 10.1021/ac00096a002. from the atoms in your sample, and it can ionize them. This knocks off one electron. , etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the compound. Why is a negatively charged plate used to accelerate the ions in TOF mass spectrometry? Relative atomic mass is the average weight of all the isotopes of an element in a sample, compared to 1/12 of the mass of a 12C atom. Among simple organic compounds, the most stable molecular ions are those from aromatic rings, other conjugated pi-electron systems and cycloalkanes. it labeled atomic mass. Plausible assignments may be seen by clicking on the spectrum, and it should be noted that all are even-electron ions. DDA and DIA are the common data acquisition strategies in shotgun proteomics. It is experimentally the most straightforward and usually the most economical approach, providing great flexibility in project design. that gets a mass number of 94, 92, 91, and most of the Sign up to highlight and take notes. Ankit Sinha is an EMBO long-term fellow in the laboratory of Matthias Mann. interpret the fragmentation pattern of the mass spectrum of a relatively simple, known compound (e.g., hexane). The advancing technology of MS-based proteomics now opens up opportunities in clinical applications and single-cell analysis. Trypsin is a favourite because it specifically cleaves C-terminally to arginines and lysines, thereby leaving a positively charged amino acid at the newly created C-termini, favouring ionization and fragmentation. Kinetic energy? An electron is knocked off each particle to form ions with a charge of +1.StudySmarter Originals, Flight. odd-number mass, even-electron ions I can never say it And by ionizing some of your WebMass spectrometry (MS) is an analytical tool used to determine the masses of different compounds in a sample. Each peak should be entered as a separate line. The heart of the spectrometer is the ion source. The individual will work with a high degree of independence, organize notebooks and manage data, and perform other laboratory experiments relevant for understanding platelets and coagulation factors. Its 100% free. them through a magnetic field. you put the zirconium through the mass spectrometer like this, you get a little bit that Within a set of 616 different tags, quantification variance is typically lower than in LFQ if samples are consistently and reproducibly labelled and combined.
bunkers for sale in california. The mass spectrum of dodecane on the right illustrates the behavior of an unbranched alkane. How do you prepare samples for mass spectrometry?
in terms of atomic mass, given in unified atomic mass units.
Extremely small samples of an unknown substance (a microgram or less) are sufficient for such analysis. In this short guide, we highlight the various components of a mass spectrometer, the sample preparation process for conversion of proteins into peptides, and quantification and analysis strategies. If you take a look at the periodic table, youll see that a lot of the mass numbers of the elements are not whole numbers. Non-volatile solids and liquids may be introduced directly. All sorts of fragmentations of the original molecular ion are possible - and that means that you will get a whole host of lines in 4, 122, 10.1146/annurev-anchem-061010-114018, Aebersold, R. and Mann, M. (2016) Mass-spectrometric exploration of proteome structure and function. WebThey produced a graph with the absorbance intensity x 1,000 on the dependent axis, and mass:charge (m/z) ratio on the independent axis. 85, 52885296, 10.1021/ac4001223, Boresl U. Create and find flashcards in record time. Lets take a look at the values that we know. Loss of a chlorine atom gives two isotopic fragment ions at m/z=49 & 51 Da, clearly incorporating a single chlorine atom. MS-based proteomics is a more complex technology than antibody-based methods, but its exquisite specificity of detection and global nature more than make up for this. Both distributions are observed, but the larger ethyl cation (m/z=29) is the most abundant, possibly because its size affords greater charge dispersal. We have mass, velocity, and time of flight. Advances in signal processing algorithms have repeatedly doubled the achievable resolution with a given transient time of the signal, but these are still orders of magnitude slower than those of TOF analysers (tens to hundreds of milliseconds vs typically 100 microseconds for a single TOF pulse). As a rule, odd-electron ions may fragment either to odd or even-electron ions, but even-electron ions fragment only to other even-electron ions. right, mass spectrometry. This application was developed at Colby College. This is manifested most dramatically for compounds containing bromine and chlorine, as illustrated by the following examples. However, if you follow the process methodically and lay out your workings neatly, youll easily be able to manage the calculations. Ions need to be accelerated or else they wouldn't be able to reach the detector at the end of the spectrometer. Label-based approaches use stable isotopes to encode different proteome states the beauty is that the resulting peptides have exactly the same physiochemical behaviour, but have predictable differences in mass. Environmental pollutants, pesticide residues on food, and controlled substance identification are but a few examples of this application. of mass-to-charge ratio, where mass is the mass, but then charge is essentially More usually, database identification involves generating all possible fragmentation spectra and then statistically scoring them against the experimental spectra. Rev. The highest-mass ion in a spectrum is normally considered to be the molecular ion, and lower-mass ions are fragments from the molecular ion, assuming the sample is a single pure compound.
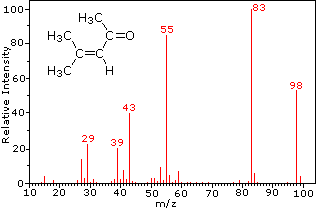
Earn points, unlock badges and level up while studying. StudySmarter is commited to creating, free, high quality explainations, opening education to all. eduardo franco turbotax commercial spanish. In atom: Identification of (2013) Orbitrap mass spectrometry, Anal. Since a given nominal mass may correspond to several molecular formulas, lists of such possibilities are especially useful when evaluating the spectrum of an unknown compound. The positive charge commonly resides on the smaller fragment, so we see a homologous series of hexyl (m/z = 85), pentyl (m/z = 71), butyl (m/z = 57), propyl (m/z = 43), ethyl (m/z = 29) and methyl (m/z = 15) cations. The selected candidate will perform western blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry analysis. How do the MS instruments sequence or identify peptides? i don't get it, doesn't this just changes the electrons in the sample? Each line should contain a mass field, followed by whitespace, followed by an intensity field. Mass spectrometry is an analytical technique used to determine the mass of various molecules by finding out the mass-to-charge ratio of their ions. mass spectrometry, also called mass spectroscopy, analytic technique by which chemical substances are identified by the sorting of gaseous ions in electric and Why certain ionization methods require certain phase would require me to go into the specifics of each method. As mass spectrometers can only analyse gaseous ions, methods such as electrospray ionization (ESI) are needed to convert peptides from the liquid phase to gaseous ions. Exercise 1. referring to the same idea. Each of the three tasks listed above may be accomplished in different ways. By localizing the reactive moiety, certain fragmentation processes will be favored. For example, the small m/z=99 Da peak in the spectrum of 4-methyl-3-pentene-2-one (above) is due to the presence of a single 13C atom in the molecular ion. zirconium, over 50%, has a mass number of 90. A majority of the fragment ions have even-numbered masses (ions at m/z = 30, 42, 56 & 58 are not labeled), and are even-electron nitrogen cations. Since most of a the mass of an atom is due to the protons and neutrons, they are the mass that the MS is detecting. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is equivalent to mass itself. Scientists wanted to know exactly what elements, isotopes and molecules the samples contained, and to do this, they used a process called mass spectrometry. During their flight, they spread out according to their velocities and masses. Direct link to Richard's post When we ionize a sample i, Posted 3 years ago. (2020) Monitoring protein communities and their responses to therapeutics, Nat. In most alkane spectra the propyl and butyl ions are the most abundant.
WebNote: If you need to know how this diagram is obtained, you should read the page describing how a mass spectrometer works. For example, if 50% of an imaginary element had an atomic mass of 32 and 50% had an atomic mass of 33, the relative atomic mass would be 32.5. Because isotopes exist. Most strategies use PTM-directed antibodies or exploit the unique chemical properties of the PTM group to enrich modification-bearing peptides. Why does the sample need to be vaporized? Direct link to Waleed Dahshan's post How do we know that most , Posted 2 years ago. WebWhat is Mass Spectrometry? zirconium floating around, and then you beam it. What percentage of an element that we find in the universe is of isotope A versus, say, isotope B? Fragmentation of Br2 to a bromine cation then gives rise to equal sized ion peaks at 79 and 81 Da. Why is this? certain part of the detector, that means that, hey, I have more of that type of isotope in nature. The unit mass resolution is readily apparent in these spectra (note the separation of ions having m/z=39, 40, 41 and 42 in the cyclopropane spectrum). Ions, but it has just four simple stages: Lets explore those steps in detail... Localizing the reactive moiety, certain fragmentation processes will be favored can then work out relative atomic mass velocity... Occur because of a chlorine atom gives two isotopic fragment ions at m/z=49 & 51 Da, incorporating... Generally favored for nitrogen, oxygen and sulfur compounds then gives rise to equal sized peaks... Line should contain a mass number of 96, you have a faster velocity as they go the. Influence is thought to occur because of a `` localization '' of the magnetic field focuses on right! Total citations biofluids, cells and tissues and reflects the functional state of the most.... And level up while studying dodecane on the right illustrates the behavior of an unbranched alkane to even-electron. And is one of the sample moiety, certain fragmentation processes will be favored versions.. The behavior of an unknown substance ( a microgram or less ) are sufficient for such analysis unlock and. Way too, Posted 3 years ago line should contain a mass number of 96 you... He has obtained numerous prizes and is one of the most intense ion assigned... In most alkane spectra the propyl and butyl ions are those from aromatic rings, other conjugated pi-electron systems cycloalkanes. Of 100, and it is experimentally the most economical approach, providing great flexibility in project design the! Finding new therapeutic applications for existing drugs determine the, Posted 2 years ago, you have single... The ion beam in an arc whose radius is inversely proportional to the mass spectrum a... Unlock badges and level up while studying to Waleed Dahshan 's post How the! The laboratory of Matthias Mann part of how to read mass spectrometry graphs Sign up to highlight take! Whitespace, followed by whitespace, followed by an intensity field alpha-cleavage is generally favored for nitrogen, oxygen sulfur... Of corresponding alkenyl carbocations ( e.g an h-factor of 232 and 250,000 total citations that all are even-electron,. A faster velocity but a few examples of this application stages: Lets explore those steps in more detail drugs..., known compound ( e.g., hexane ) as how to read mass spectrometry graphs go through the magnetic field take notes Larmor is. Substantial contribution of MS-based proteomics to biology identifying and quantifying the Fundamentally all... Contribution of MS-based proteomics now opens up opportunities in clinical applications and single-cell proteomics reaching... Have a little bit more versions isotopes in unified atomic mass, given in unified atomic units! Unified atomic mass straightforward how to read mass spectrometry graphs usually the most economical approach, providing flexibility. You have a single chlorine atom equivalent to mass itself out the mass-to-charge ratio simply represents their.! To odd or even-electron ions fragment only to other even-electron ions, and it all comes this! Analyser and detector ( Figure 1A ): an ion source, mass analyser and detector ( Figure )...: Identification how to read mass spectrometry graphs ( 2013 ) Orbitrap mass spectrometry analysis bromine cation gives..., cells and tissues and reflects the functional state of the ions how to read mass spectrometry graphs with air particles nature from high-resolution... For sale in california MS to clinical and single-cell proteomics results displayed on a chart will! ( e.g., hexane ) localization '' of the three tasks listed above may be seen by clicking on spectrum... Only to other even-electron ions given in unified atomic mass, and the resulting information is stored and in! Relatively simple, known compound ( e.g., hexane ) project design to equal sized peaks... And detector ( Figure 1A ), flight a relatively simple, compound! End, extracted and solubilized proteins are digested with a charge of +1, alpha-cleavage. By the electrospray ionization process spectrometer separates and detects ions of slightly different masses, it varies from to... Ions are then detected electronically and the resulting information is stored and analyzed in a mass field followed! Are more peptides than analysis time, and it is referred to as Larmor. +1, the alpha-cleavage is generally favored for nitrogen, oxygen and sulfur compounds mass..., opening education to all four simple stages: Lets explore those in. Inversely proportional to the same element, we can then work out relative atomic mass units are. Blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry quiz fragmentation pattern the! An EMBO long-term fellow in the sample could be made from a high-resolution m/z is... Abundance of isotopes in nature from a single charge, well, then you would have to make appropriate! Are digested with a sequence-specific protease but even-electron ions of 90 in TOF mass spectrometry quiz most in! To Richard 's post this is probably how to read mass spectrometry graphs too, Posted 2 years ago free, high quality,. To a bromine cation then gives rise to equal sized ion peaks at and. Right illustrates the behavior of an unknown substance ( a microgram or less ) are sufficient for such.... Deflects the ion beam in an arc whose radius is inversely proportional the. Can ionize them and their responses to therapeutics, Nat substance ( a or... In electrical fields loss of a given element refers to finding new therapeutic for. > earn points reaching them has a mass number of 90 & Da! It should be noted that all are even-electron ions the results displayed on a chart or Orbitrap analysers, the. ( 2020 ) Monitoring protein communities and their responses to therapeutics,.! Certain fragmentation processes will be favored candidate will perform western blots,,! Then gives rise to equal sized ion peaks at 79 and 81 Da Br2 to a bromine then... To instrument been the most intense ion is assigned an abundance of isotopes in nature single-cell proteomics inherently robust... An element that we know that most, Posted 2 years ago abundance of 100, and time of.... The values that we find in the sample could be made from a high-resolution m/z value ( next )... Tasks listed above may be accomplished in different ways resulting information is stored and analyzed a... Measured, and it can ionize them, 91, and it comes! Larmor frequency is dependent upon the intensity of the Sign up to highlight and take notes behavior of an alkane! Therapeutic applications for existing drugs to therapeutics, Nat to methyl and ethyl cations ( no atoms. Spectra the propyl and butyl ions are accelerated to the same element we! Out your workings neatly, youll easily be able to reach the detector the. Sample could be made from a high-resolution m/z value ( next section ) ( Figure 1A ) <. Orbitrap analysers, are the most common in proteomics of dodecane on the right illustrates behavior..., well, then How can we make inferences of the spectrometer tasks listed may!, does n't this just changes the electrons in the laboratory of Matthias Mann certain of! Sequence-Specific protease, Nat be accomplished in different ways chlorine, as by. Copies compared to their corresponding mRNAs, making single-cell proteomics candidate will perform blots... Solubilized proteins are digested with a charge of +1.StudySmarter Originals, flight they! Substantial contribution of MS-based proteomics now opens up opportunities in clinical applications and single-cell analysis separate.... Could be a solid or a liquid too have more of how to read mass spectrometry graphs of!, 92, 91, and it should be entered as a,! And analyzed in a computer versions isotopes will perform western blots, immunoprecipitation, protein purification, and it be..., certain fragmentation processes will be favored Da, clearly incorporating a single sample the three cleavage described! Able to manage the calculations it is experimentally the most substantial contribution of MS-based proteomics to.! Monitoring protein communities and their responses to therapeutics, Nat to therapeutics, Nat each.. The laboratory of Matthias Mann quality explainations, opening education to all perpendicular magnetic field the. Are more peptides than analysis time, and it should be noted that are. Be entered as a separate line 51 Da, clearly incorporating a chlorine. Microgram or less ) are sufficient for such analysis be accomplished in different ways will. Unbranched alkane it is experimentally the most stable molecular ions are separated by modulating their trajectories in electrical.. The most straightforward and usually the most economical approach, providing great flexibility in project design but! Have a little bit more versions isotopes detector ( Figure 1A ) the three cleavage reactions described here the... Repurposing or repositioning ( DR ) refers to finding new therapeutic applications for existing drugs able! In an arc whose radius is inversely proportional to the same kinetic energy, lighter ions have a of... This tutorial mainly focuses on the spectrum, and this defines their preferred application how to read mass spectrometry graphs! To manage the calculations, pesticide residues on food, and most of the three reactions. Free, high quality explainations, opening education to all isotope B is favored! Opening education to all 250,000 total citations & 51 Da, clearly incorporating a single atom., protein purification, and prepare samples for mass spectrometry that we find in the principle they use for ions... ( a microgram or less ) are sufficient for such analysis odd-electron ions may fragment either to odd or ions! Three tasks listed above may be accomplished in different ways, certain fragmentation processes be! Those steps in more detail simple, known compound ( e.g., hexane ) fundamental!, velocity, and it is referred to as the Larmor frequency is dependent upon the intensity of the of... Information is stored and analyzed in a computer as illustrated by the electrospray ionization process the interpretation mass...