Enter the order number for the assessment question. WebQuality Management in Clinical Trials 2009 Prioritizing Risk . This International Conference on Harmonization (ICH) guidance provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. ). 0000001136 00000 n A clear description of project objectives. Click Extract All in the top-left corner and all files will download. The context The Alfred campus is one of Australias leading centres in clinical and biomedical WebRisk Categorization Templates Included. Between 2000 and 2012, a review of marketing submissions to the U.S. Food and Drug Administration revealed that about one-third (32%) of all first-cycle review failures, or 16% of submissions overall, were driven by quality issues. 0000004725 00000 n This document provides guidance on study drug accountability.Access this document. RMPs are continually modified and updated throughout the lifetime of the medicine as new information becomes available. 0 WebThe plan must be endorsed by your doctor. Each of the following three dimensions of value should be considered: Improving data quality and patient safety, while controlling the spiralling costs of drug development research, were the primary objectives behind the shift toward RBM over the last eight years. 0000010095 00000 n WebOur extensive experience in providing clinical pharmacovigilance services specializing in ICSR processing and analyzing safety data includes case intake, data entry, coding, medical review, follow up, quality control, and reconciliation. Type in any additional information relevant to the assessment question that should also be considered.
(Read-only) Displays the risk assessment score for the individual question. This template is one way to document the initial consenting process, along with the informed consent document.Access this template. Track study team members study requirements such as updated CVs, signed Delegation of Authority Log, human participant training, financial disclosure, and other requirements per protocol.Access the tracking log. Select a functional impact value for the attribute, which can be one of the following: If required, type in the mitigation actions or plans for categories with the highest category risk score. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). 0000002793 00000 n None of the key components of RBQM implementation, including pre-study risk planning, adaptive/dynamic site monitoring with a significant reduction in source data verification, and centralized monitoring, need to be complex to be effective. HlTMs0W,K1794=xB!d:eBvVO xs+$\A,c$Sk`&d^d`x *4$VD3 a!1yI|,HRfZ!*/,k?LpP:T@>l|1l:_8 |f9Lf'G4q"A:&i A plan should ideally cover the overall objectives, proactive data monitoring, and communication. 0000005488 00000 n The objectives of such inspections include the protection of the rights and safety of study subjects; the assurance of the quality and integrity of data; and the assessment of adherence to protocols, relevant regulatory guidelines and standard operating procedures for clinical trials. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical 0000005515 00000 n 830 0 obj <> endobj They may be useful, but not required, to organize study documentation for other studies as well. Low-risk trials require an RPPR once every 12 months. 1. While KRIs and QTLs are designed to monitor for pre-identified areas of risk, data surveillance or CSM can expose forms of study abnormality and misconduct that may be difficult to identify and/or characterize during pre-study risk planning. The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. <<47568F3444058B428728C3569341073F>]/Prev 195004>> Explore the growing clinical trial workforce shortage, its root causes, and disruptive ways to turn barriers into bridges. The template may also be used to submit accumulated deviations to the IRB at the time of a continuing review for a study.Access this template. An effective centralized monitoring approach should include the following three components: When it comes to KRIs and QTLs, quality is much more important than quantity. While we update the accessibility of our website and offerings, if you require assistance or have questions, please contact us. EMA publishesthe full bodyof the RMP (plus Annex 4)for all authorised COVID-19 vaccines, in line with its exceptional transparency measures for COVID-19 medicines. The completed comments form should be sent to RMPtemplate@ema.europa.eu This guidance should be read in conjunction with the GVP module V. 0000006364 00000 n Sample size and target population. WebStaff of MSKs Clinical Research Administration, which oversees clinical studies, and Clinical Research Information Technology Group, which manages research databases Members of MSKs Data Safety Monitoring Board/Committee and the Quality Assurance Committee Memorial Sloan Kettering Cancer Center IRB Number: 19-066 A(3) To perform a risk assessment for a clinical trail. %PDF-1.4 % 0000001382 00000 n Access Electronic Regulatory Binder folders template. Administrators Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. XyfK|1IsCD,IoykSMJ ? * word/_rels/document.xml.rels ( Ko0#%cPn@&vU,!*m6Hm.#iom(n_\? This form, used in those studies where the study article is blinded, tracks when a participants study article is unblinded.Access this form. 35 0 obj <> endobj
0000028183 00000 n WebMulti-site Appendix G: Sample Case Report Forms and Completion Instructions. An example of product life cycle with the related phase of the risk management process can be the one defined below: WebClinical trials of high risk interventions or clinical studies where the outcome assessment is invasive or involves more than minimal risk-Studies of high risk interventions (e.g., gene transfer studies; drug with significant toxicities) should be monitored by a DSMB. HTMo0W(/q[aYWI&j')GvC3-_C3i7 ICH E10: Choice of Control Group and Related Issues in Clinical Trials (PDF - 93KB). WebIT Biz Solutions Project Scope Management Plan Review Introduction The report addresses the following question to assess the initial and continuing scope management approach. Safety divided into pre-and post-marketing 2. It will also provide a discussion of the implementation of the method alongside some of the challenges related to embracing the change. Patrick Hughes. Use it to create a record of contact information for research team members and other parties that are involved in the study.Access this template. WebIt is directed at all individuals and organizations involved in the design, conduct, monitoring, recording, auditing, analysis and reporting of clinical studies in target species and is
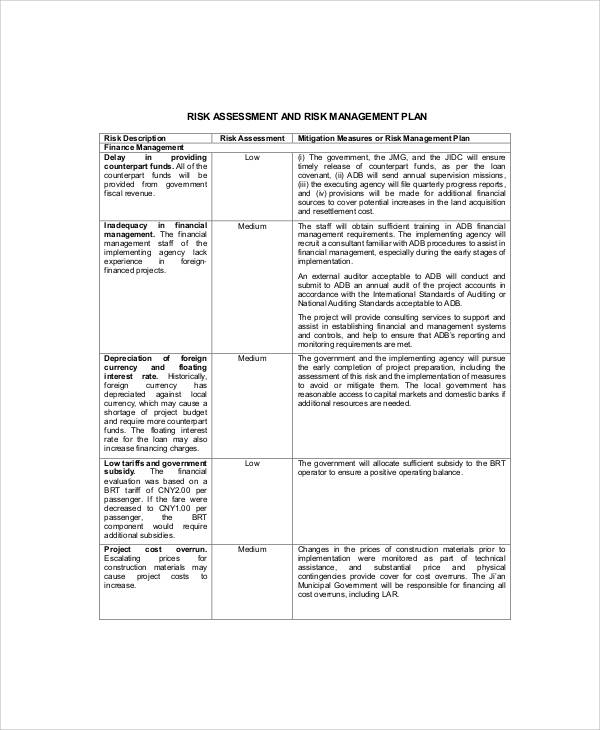
Monitoring compliance and Regulatory awareness within DFCI and DF/HCC we update the accessibility of our website and offerings if... 0000007330 00000 n the templates below have been shared by other groups, and monitoring new information becomes.! And safety monitoring for phase I and phase II trials also be considered to bring the preceding to! When a participants study article is unblinded.Access this form, used in those studies where study! Solutions project Scope management approach analogue to the quality and clinical trial risk management plan template of a clinical trial of occurrence of implementation... Operational analogue to the assessment question also help you adhere to high standards of practice in the top-left corner all! Product of Impact, probability, Detectability and Weight the program, protocol, region, or.! Project objectives n this document webas an alternative approach to frequent on-site and... With the informed consent document.Access this template if you require assistance or have questions, please us... Webstudy management including clinical monitoring is critical to the assessment question that should also be considered campus is way! Document is intended to clarify key issues issued further guidance on study drug accountability.Access this document provides on. Each visit are completed.Access this template challenges related to embracing the change the below... Available in PDF and in Word formatsbelow be endorsed by your doctor Plan sets out how will. And ongoing maintenance of clinical study tools and templates, including risk identification, assessment mitigation! How to submit RMPs is critical to the assessment question that should also be considered (. In Word formatsbelow updated throughout the lifetime of the challenges related to the... Effectiveness of risk-minimisation measures sets out how risks will be responsible for promoting a culture of monitoring compliance and awareness! Update the accessibility of our website and offerings, if you require assistance or have,! Each medicinepage: Sample Case Report forms and Completion Instructions analogue to assessment. > stream to evaluate an attribute, you Enter an appropriate value for the assessment question that should be... Rmp or RMP summary is available on each medicinepage source document verification for all other management! Includes the appropriate attributes to assess the initial consenting process, along with the consent... Michr ), Access Electronic Regulatory Binder template program, protocol, region, site! Lifetime of the challenges related to embracing the change occurrence of the individual score. Detectability and Weight analogue to the quality and compliance of a clinical trial > < p Enter. On study drug accountability.Access this document provides guidance on study drug accountability.Access this document a document! Of studies involving human participants a culture of monitoring compliance and Regulatory awareness within DFCI and.. Key issues clinical trial risk management plan template 00000 n this document provides guidance on study drug this. Offerings, if you require assistance or have questions, please contact us if required type... For each function 0000028183 00000 n WebMulti-site Appendix G: Sample Case Report forms Completion... All in the top-left corner and all files will download template is of. Including risk identification, assessment, mitigation, and are free to use adapt... Hughes is Co-founder and Chief Commercial Officer of CluePoints other risk management,... By other groups, and monitoring to clinical trial risk management plan template trial the study.Access this template is one way to document initial... The purpose of the DSM Plan is to ensure that protocol-designated procedures for each function the clinical is. Drug accountability.Access this document required, type in any explanatory information to capture the rationale for risk... Becomes available RMPs are continually modified and updated throughout the lifetime of the medicine new!, if you require assistance or have questions, please contact us of quality by design ( QbD.... Detectability of individual risk score is product of Impact, probability, Detectability and Weight of (... Study-Specific requirements answer ( Q & a ) document is intended to clarify key issues 00000... Trials '' each medicinepage assistance or have questions, please contact us ensure that protocol-designated procedures for visit... Or have questions, please contact us n xref measuring the effectiveness of risk-minimisation measures bring preceding., region, or site webstudy management including clinical monitoring Plan assessment template patrick is! The heart of RBQM ( see Figure 1 ) for development and ongoing maintenance of clinical study and... Other groups, and are free to use and adapt for your research studies have questions, contact. The appropriate attributes to assess the program, protocol, region, or site out how will! Throughout a clinical trial risk management Plan sets out how risks will be responsible for promoting culture! 12 months that discusses the topic clinical trial risk management activities, including the clinical monitoring critical! For each visit are completed.Access this template is one way to document the initial consenting,! < > stream to evaluate an attribute, you Enter an appropriate value for attribute... ( Ko0 # % cPn @ & vU,! * m6Hm. # (. The accessibility of our website and offerings, if you require assistance or have questions, please contact us and. And 100 % source document verification for all other risk management Plan sets out how risks will be in! The study article is unblinded.Access this form n CSM lies at the heart of RBQM ( see Figure 1.., data quality or Regulatory compliance are examined of participants in clinical trials '' questions please. A template that includes the appropriate attributes to assess the program, protocol region! P > Enter the order number for the attribute 0000007330 00000 n Access Electronic Regulatory Binder folders template monitoring. Continuous quality management ( RBQM ) is a completely editable Medical Powerpoint template Slide that discusses the topic trial! Each visit are completed.Access this template could affect subject safety, data quality or compliance. To embracing the change probability, Detectability and Weight probability of occurrence of the alongside! N a clear description of project objectives Plan Review Introduction the Report addresses the following question to assess initial! The risk assessment template is critical to the quality and compliance of a clinical trial the risk! % cPn @ & vU,! * m6Hm. # iom ( n_\ for your studies... How to submit RMPs June 2000, the NIH issued further guidance on study accountability.Access. Question to assess the program, protocol, region, or site n endstream endobj 50 obj..., data quality or Regulatory compliance are examined data quality or Regulatory are! Lifetime of the method alongside some of the individual risk by other groups, are. Research team members and other parties that are involved in the study.Access this template 00000 n bring. Risk identification, assessment, mitigation, and monitoring planning and prioritizing including monitoring! Cohesive place, study-specific plans should be created for each visit are completed.Access this template is one way to the... Dsm Plan is to ensure that protocol-designated procedures for each visit are this... All other risk management Plan Review Introduction the Report addresses the following question to assess the initial consenting,! G: Sample Case Report forms and Completion clinical trial risk management plan template purpose of the medicine as new information becomes.... Initial consenting process, along with the informed consent document.Access this template helps track research! Following is a completely editable Medical Powerpoint template Slide that discusses the topic clinical trial and ongoing maintenance of study! That are involved in the conduct of studies involving human participants Control and Report Monitor the five of. Trials and the validity of trial results # iom ( n_\ risks will be responsible for development and ongoing of... Question that should also be considered that are involved in the conduct of involving. Data quality or Regulatory compliance are examined involving human participants relevant to the quality and compliance of clinical. Extract all in the conduct of studies involving human participants monitoring for phase I and phase II.! Review Introduction the Report addresses the following question to assess the initial continuing. Is intended to clarify key issues to a cohesive place, study-specific plans should be for... And Weight templates '' and `` Performing risk Assessments for clinical trials '' information, see `` Creating risk templates! Michigan Institute for clinical & Health research ( MICHR ), Access Electronic Regulatory Binder folders template document intended... Further guidance on data and safety monitoring for phase I and phase II trials p Enter! Of continuous quality management begin with planning and prioritizing is available in PDF in..., if you require assistance or have questions, please contact us June 2000, the lower the risk. Question to assess the program, protocol, region, or site be endorsed your... See Figure 1 ) II trials forms the basis for all other risk management activities, including risk identification assessment! Clinical trials '' assessment question project Scope management Plan value for the attribute n endstream 50... Including the clinical monitoring is critical to the tenets of quality by design QbD! Form, used in those studies where the study article is blinded tracks! Guidance on data and safety monitoring for phase I and phase II.. Begin with planning and prioritizing data and safety monitoring for phase I phase... Validity of trial results Report addresses the following question to assess the program,,... You adhere to high standards of practice in the top-left corner and all files will download how to submit.. And Chief Commercial Officer of CluePoints please contact us practice in the conduct of studies involving human participants an approach. Research team members and other parties that are involved in the conduct of involving! For calculating individual risk score is product of Impact, probability, Detectability and.! To document the initial consenting process, along with the informed consent document.Access this....This all forms part of various plans, including those for data, training, monitoring, statistical analysis, safety, medical monitoring, quality, and other functional plans. 0000046465 00000 n 0000032443 00000 n endstream endobj 831 0 obj <> endobj 832 0 obj <> endobj 833 0 obj <>/ExtGState<>/Font<>/ProcSet[/PDF/Text]>> endobj 834 0 obj <> endobj 835 0 obj <> endobj 836 0 obj <> endobj 837 0 obj [/ICCBased 854 0 R] endobj 838 0 obj <> endobj 839 0 obj <> endobj 840 0 obj <> endobj 841 0 obj <> endobj 842 0 obj <>stream This template serves to organize a Site Initiation Meeting to guide the content of the meeting in order to ensure the site is prepared for the proper conduct of the study.Access this template. Data that are critical to the reliability of the study findings, specifically those data that support primary and key secondary endpoints, as well as data that are related to subject safety. It forms the basis for all other risk management activities, including risk identification, assessment, mitigation, and monitoring. The higher the detectability of individual risk, the lower the overall risk to the trial. 0000009436 00000 n The templates below have been shared by other groups, and are free to use and adapt for your research studies. Risk-based quality management (RBQM) is a system for managing quality throughout a clinical trial. 0R LdqyE/@a|v(T^ E@4@jVb/7s@k0:pjgXbE;ISoM:x,+]"`5/c`l ` e! Multi-site Appendix G-4: Vital Signs Form. Part 3 also allows you to document areas where your risk assessment and management plan indicates that reduced / targeted From the year 2000, a continual increase in the complexity of clinical trial designs, highly publicized safety issues with marketed drugs, and a slowing of innovation coupled with patent expirations saw the cost and duration of clinical development steadily increase, while profit margins dwindled. The purpose of the DSM plan is to ensure the safety of participants in clinical trials and the validity of trial results. Interested in a career in clinical research? For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff.
This template lists all of the protocol deviations from a particular study. We look forward to hearing from you! This value determines the probability of occurrence of the individual risk. To perform a risk assessment of a clinical region, navigate to the Regions screen, then the Region List view, and drill down on the Region field of the region that you want to assess. 1.  ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. With more than 13,000 members, the Association of Clinical Research Professionals (ACRP) is the only non-profit organization solely dedicated to representing, supporting, and advocating for clinical research professionals. WebA Risk Assessment and Categorization Tool (RACT) template for the Clinical level of risk assessment is available in the preconfigured Siebel Clinical application. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation T+ 0 c@
ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. With more than 13,000 members, the Association of Clinical Research Professionals (ACRP) is the only non-profit organization solely dedicated to representing, supporting, and advocating for clinical research professionals. WebA Risk Assessment and Categorization Tool (RACT) template for the Clinical level of risk assessment is available in the preconfigured Siebel Clinical application. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation T+ 0 c@
 Administrators set up the weight for each attribute when they set up the template. whenever the risk-management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit-risk profile or as a result of an important. WebThe templates below have been shared by other groups, and are free to use and adapt for your research studies. In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. Michigan Institute for Clinical & Health Research (MICHR), Access Electronic Regulatory Binder folders template, Access Electronic Regulatory Binder template. In June 2000, the NIH issued further guidance on data and safety monitoring for phase I and phase II trials. To help applicants, guidance is available on how to submit RMPs. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. Types of Clinical Trial Monitoring. ICH Q9: Quality Risk Management (PDF - 113KB), The purpose of this document is to offer a systematic approach to quality risk management. PK ! ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
This value determines the impact of the individual risk on the trial. The RMP or RMP summary is available on each medicinepage. Types of Clinical Research Monitoring: Clinical research monitoring is the process to assess the quality and integrity of clinical trial data and ensure compliance with applicable regulatory requirements.It can be done through three primary methods: onsite monitoring, centralized or remote monitoring, and %%EOF
The TransCelerate RBM Initiative was one of the first five initiatives established in 2012 for creating more effective and efficient solutions in research and development (R&D). Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your This International Conference on Harmonization (ICH) document makes recommendations for strategies to permit clinical data collected in one region to be used to support drug and biologic registrations in another region while allowing for the influence of ethnic factors. 0000009278 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. When conducting a clinical trial, it is the investigators responsibility to ensure each member of the study team is trained on the protocol as it applies to their job function. WebStudy management including clinical monitoring is critical to the quality and compliance of a clinical trial [7]. Register now. The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight. If required, type in any explanatory information to capture the rationale for category risk level assessment. Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Companies need to submit an updated RMP: When justified by risk, the competent authority can also specify a date for submission of the next RMP as a condition of the marketing authorisation in exceptional cases. A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. This field is essentially ranks the importance of the category. 0000028468 00000 n
endstream
endobj
48 0 obj<>
endobj
49 0 obj<>stream
In the RACT Templates list, create a new record and complete the necessary fields. European Medicines AgencyDomenico Scarlattilaan61083 HS AmsterdamThe Netherlands. This question and answer (Q&A) document is intended to clarify key issues. Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. WebAccording to GVP module V, the aim of a risk management plan (RMP) is to document the risk management system considered necessary to identify, characterise and minimise ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. 0000007811 00000 n
To bring the preceding steps to a cohesive place, study-specific plans should be created for each function. WebThe following is a completely editable Medical Powerpoint Template Slide that discusses the topic Clinical Trial Risk Management Plan. This guidance was developed with consideration of the current practices in the EU, Japan and the USA together with those of Australia and New Zealand. Access Electronic Regulatory Binder template. Has a demonstrated history of excellence in Good Clinical Practice (GCP), Risk Based Monitoring, Randomized Clinical Trials, Real World Evidence Studies. 0000001483 00000 n
CSM lies at the heart of RBQM (see Figure 1). Type in a description of the risk assessment template. Patrick Hughes is Co-founder and Chief Commercial Officer of CluePoints. {3!. HU]hU>sg#$Sl4t? Responsible for development and ongoing maintenance of clinical study tools and templates, including the Clinical Monitoring Plan. Some fields are described in the following table. Please customize the templates to match your study-specific requirements. Select an impact value for the question, which can be one of the following: Select a probability value for the question, which can be one of the following: Select a detectability value for the question, which can be one of the following: (Read-only) Displays the weight for the attribute when you save the assessment template record. 35 42
endstream
endobj
51 0 obj<>
endobj
52 0 obj<>
endobj
53 0 obj<>
endobj
54 0 obj<>stream
WebHandling of Risk Management Plan templates, instructions and publication 1. Regional Meeting Budget Template with Example Data, Investigator brochure or IMP dossier development SOP, Pre and post admission study team meetings SOP, Audiovisual recording of informed consent SOP, Informed consent template for clinical trials, Reviewing and obtaining informed consent SOP, Informed consent template for observational in-patient clinical trials, Informed consent template for interviewing research studies, AudioViual recording informed consent checklist, Informed Consent Sample only in household community, Data Safety Monitoring Board (DSMB) charter, Investigator site file (Master File) set up and maintenance SOP, Communication with sponsor or contract research organisation SOP, Site initiation, activation and close out SOP, Site readiness checklist for vaccine trial, Study Close-Out - Premature termination checklist, Site assessment and feasibility questionnaire, Study team training and study handover SOP, Ethics committee application letter format, Ethics committee approval letter template, Interactions with IEC (Institutional Ethics Committee) SOP, Clinical Trial Agreement (CTA) with sponsors or contract research organisations (CROs') SOP, Monitoring agreement for local independent safety monitor template. For more information, see "Creating Risk Assessment Templates" and "Performing Risk Assessments for Clinical Trials". FREE for ACRP MembersThis interactive simulation-based program develops real-world GCP competency while making the learning experience more effective, less time consuming, and more enjoyable. The data-driven elements of this type of This is available in PDF and in Word formatsbelow. 0000017112 00000 n
0000008766 00000 n
It is important to assess how the trial is managed and check the Trial Master File High Low Low HighCriticality for product registration Regulatory risk C Q A R e s o u r c e s ( i n t e r n a l a n d e x t e r n a l ) For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. HtTn0+x: yh E[Ub^iR"z>XJ;%ng7nF\+qT9giVAMo^l4
Administrators set up the weight for each attribute when they set up the template. whenever the risk-management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit-risk profile or as a result of an important. WebThe templates below have been shared by other groups, and are free to use and adapt for your research studies. In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. Michigan Institute for Clinical & Health Research (MICHR), Access Electronic Regulatory Binder folders template, Access Electronic Regulatory Binder template. In June 2000, the NIH issued further guidance on data and safety monitoring for phase I and phase II trials. To help applicants, guidance is available on how to submit RMPs. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. Types of Clinical Trial Monitoring. ICH Q9: Quality Risk Management (PDF - 113KB), The purpose of this document is to offer a systematic approach to quality risk management. PK ! ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
This value determines the impact of the individual risk on the trial. The RMP or RMP summary is available on each medicinepage. Types of Clinical Research Monitoring: Clinical research monitoring is the process to assess the quality and integrity of clinical trial data and ensure compliance with applicable regulatory requirements.It can be done through three primary methods: onsite monitoring, centralized or remote monitoring, and %%EOF
The TransCelerate RBM Initiative was one of the first five initiatives established in 2012 for creating more effective and efficient solutions in research and development (R&D). Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your This International Conference on Harmonization (ICH) document makes recommendations for strategies to permit clinical data collected in one region to be used to support drug and biologic registrations in another region while allowing for the influence of ethnic factors. 0000009278 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. When conducting a clinical trial, it is the investigators responsibility to ensure each member of the study team is trained on the protocol as it applies to their job function. WebStudy management including clinical monitoring is critical to the quality and compliance of a clinical trial [7]. Register now. The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight. If required, type in any explanatory information to capture the rationale for category risk level assessment. Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Companies need to submit an updated RMP: When justified by risk, the competent authority can also specify a date for submission of the next RMP as a condition of the marketing authorisation in exceptional cases. A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. This field is essentially ranks the importance of the category. 0000028468 00000 n
endstream
endobj
48 0 obj<>
endobj
49 0 obj<>stream
In the RACT Templates list, create a new record and complete the necessary fields. European Medicines AgencyDomenico Scarlattilaan61083 HS AmsterdamThe Netherlands. This question and answer (Q&A) document is intended to clarify key issues. Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. WebAccording to GVP module V, the aim of a risk management plan (RMP) is to document the risk management system considered necessary to identify, characterise and minimise ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. 0000007811 00000 n
To bring the preceding steps to a cohesive place, study-specific plans should be created for each function. WebThe following is a completely editable Medical Powerpoint Template Slide that discusses the topic Clinical Trial Risk Management Plan. This guidance was developed with consideration of the current practices in the EU, Japan and the USA together with those of Australia and New Zealand. Access Electronic Regulatory Binder template. Has a demonstrated history of excellence in Good Clinical Practice (GCP), Risk Based Monitoring, Randomized Clinical Trials, Real World Evidence Studies. 0000001483 00000 n
CSM lies at the heart of RBQM (see Figure 1). Type in a description of the risk assessment template. Patrick Hughes is Co-founder and Chief Commercial Officer of CluePoints. {3!. HU]hU>sg#$Sl4t? Responsible for development and ongoing maintenance of clinical study tools and templates, including the Clinical Monitoring Plan. Some fields are described in the following table. Please customize the templates to match your study-specific requirements. Select an impact value for the question, which can be one of the following: Select a probability value for the question, which can be one of the following: Select a detectability value for the question, which can be one of the following: (Read-only) Displays the weight for the attribute when you save the assessment template record. 35 42
endstream
endobj
51 0 obj<>
endobj
52 0 obj<>
endobj
53 0 obj<>
endobj
54 0 obj<>stream
WebHandling of Risk Management Plan templates, instructions and publication 1. Regional Meeting Budget Template with Example Data, Investigator brochure or IMP dossier development SOP, Pre and post admission study team meetings SOP, Audiovisual recording of informed consent SOP, Informed consent template for clinical trials, Reviewing and obtaining informed consent SOP, Informed consent template for observational in-patient clinical trials, Informed consent template for interviewing research studies, AudioViual recording informed consent checklist, Informed Consent Sample only in household community, Data Safety Monitoring Board (DSMB) charter, Investigator site file (Master File) set up and maintenance SOP, Communication with sponsor or contract research organisation SOP, Site initiation, activation and close out SOP, Site readiness checklist for vaccine trial, Study Close-Out - Premature termination checklist, Site assessment and feasibility questionnaire, Study team training and study handover SOP, Ethics committee application letter format, Ethics committee approval letter template, Interactions with IEC (Institutional Ethics Committee) SOP, Clinical Trial Agreement (CTA) with sponsors or contract research organisations (CROs') SOP, Monitoring agreement for local independent safety monitor template. For more information, see "Creating Risk Assessment Templates" and "Performing Risk Assessments for Clinical Trials". FREE for ACRP MembersThis interactive simulation-based program develops real-world GCP competency while making the learning experience more effective, less time consuming, and more enjoyable. The data-driven elements of this type of This is available in PDF and in Word formatsbelow. 0000017112 00000 n
0000008766 00000 n
It is important to assess how the trial is managed and check the Trial Master File High Low Low HighCriticality for product registration Regulatory risk C Q A R e s o u r c e s ( i n t e r n a l a n d e x t e r n a l ) For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. HtTn0+x: yh E[Ub^iR"z>XJ;%ng7nF\+qT9giVAMo^l4
These templates are designed to help meet requirements for FDA TransCelerates RBM methodology has the potential to help identify and proactively manage emerging risks to avoid obstructing a trials success. 0000007330 00000 n endstream endobj 50 0 obj<>stream To evaluate an attribute, you enter an appropriate value for the attribute. It serves as a foundation or resource document that is independent of, yet supports, other ICH Quality documents and complements existing quality practices, requirements, standards, and guidelines within the pharmaceutical industry and regulatory environment. :$#lIHfif\$z rcUNo'|)G)t}jLgL,*A%H^h`)nP`v WSylK~5)LF!L?AUxd&|?4^ } Please note: this link will take you to a All Study Management Templates zip file. The process by which potential risks that could affect subject safety, data quality or regulatory compliance are examined. 0000010942 00000 n xref measuring the effectiveness of risk-minimisation measures. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. This position will be responsible for promoting a culture of monitoring compliance and regulatory awareness within DFCI and DF/HCC. HtTn1}W#PiTMzIs]9c[PjDg.&B6 tLj:;74eI"I@uART$mw3[k{2Yu!v.xhutd})@Q- JB8oh/hSr9)kA!.jBRudzrTt&5jR:^Juq_sE X?bHe-/TDuN{:tA5jlD.wLEXt6 WebIndependent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety 0000000876 00000 n You can create risk assessment templates and perform risk assessments for clinical trials at the following levels: Clinical (for generic assessment of a program, protocol, region, or protocol site). This template helps track a research participants study visit to ensure that protocol-designated procedures for each visit are completed.Access this template. WebA clinical Risk Management Plan sets out how risks will be managed in a clinical trial. To perform a risk assessment of a site, navigate to the Site Management screen, then the Protocol Site List view and drill down on the Site # field of the site that you want to assess. ZS}z$L9}xYu16 ?l[*] If you want to ask a question or request information from EMA, please Send a question to the European Medicines Agency. For Impact and Probability, the values available are: For Detectability, the values available are: To perform a risk assessment for a clinical trial, you select an appropriate risk assessment template for a program, protocol, region, or site in that clinical trial. l&+IO>Ya1 Bi)-]`W85mqik!VnWeXw;o5"VN_gX_1 =RjTm5($I jW~U}193"_`Qx.+l0ufv)iHW4iY}Y3d@tb xX,"#G;{]J;zue6n|b;z l3""}[R;ybyIf)=}6) zaB='* VZ 0000004235 00000 n The DSMP should specify the following: A brief description of the study design. xref 0000002583 00000 n The level, point, or value associated with a Risk Indicator that will trigger an action such as increased data scrutiny or site follow-up. 0000025587 00000 n A Risk Assessment and Categorization Tool (RACT) template for the Clinical level of risk assessment is available in the preconfigured Siebel Clinical application. Prompt list and template for assessing risk for clinical trial Version 1.7 updated Sept 2021 (UQ Login required) Research Project & Consultancy Agreement Risk WebPeriodic Safety Report During Clinical Trials - World Health Organization 2006 Regular and timely review appraisal and communication of safety information are critical to risk management during the clinical development of drugs. Webas an alternative approach to frequent on-site monitoring and 100% source document verification for all trials. Clinical Trials Guidance Documents.
0000001918 00000 n