Write the molecular formula for each compound. co N4Se4 The ionic charge of oxygen is -2. You can tell because oxygen is in group number 6, so it has 6 valence electrons. Since it needs 2 more electrons It is utilized for manufacturing phosphorus oxychloride by reacting it with oxygen. potassium sulfide, A:To write the formula of the compound , we have to identify the atoms present in the compound . Write a formula for the ionic compound that forms from each pair of elements. FePO3 Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. It reacts violently with water. It is a red solid. [7] It is also a component of some amorphous solid electrolytes (e.g. What is the name of the simplest organic compound? ClO2-, Q:how to interpret a chemical formula to identify how many molecules are represented AND how many, A:Chemical Formula is the way of representation of number of different kind of atoms in the compound., Q:Give the name for each of the following binary compounds ofcarbon: (a) CS2, (b) CO, (c) C3O2, (d), A:Binary compounds: The elements in \(\ce{Na_2O}\) are a metal and a nonmetal, which form ionic bonds. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, Name Formula A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula. In writing name of these, Q:Complete the following table: The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. The name for P3H is triphosphorus monohydride, and the name for P2H3 is diphosphorus trihydride. Nitrogen monoxide (NO) will be a covalently bound molecule (two non-metals), silicon dioxide (SiO2) will be a covalently bound molecule (a semi-metal and a non-metal) and MgCl2 will be ionic (a metal and a non-metal). PCl3 must also behave as an electron pair. Q:Write chemical formulas when given the name . Phosphorus is usually known as phosphate in minerals. (b) An ionic compound always has at least one metal.  sodium hydrogen carbonate For example, pyrite, which is also called fools gold owing to its brassy yellow colour, is a sulfide of iron with the formula FeS2. Express your answer as a chemical formula. It is used to manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and thio-phosphoryl chloride. It is not easily soluble in water. name A:Argon and Helium both are inert gases at room temperature. Consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form is far more reactive chemically than the others. Determine whether the metal in the ionic compound Cr2O3 forms only one type of ion or more than one type of ion and name the compound accordingly. Author of.
sodium hydrogen carbonate For example, pyrite, which is also called fools gold owing to its brassy yellow colour, is a sulfide of iron with the formula FeS2. Express your answer as a chemical formula. It is used to manufacture chlorinated elements like phosphoryl chloride, phosphorous Penta chloride, pseudo-halogens, and thio-phosphoryl chloride. It is not easily soluble in water. name A:Argon and Helium both are inert gases at room temperature. Consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form is far more reactive chemically than the others. Determine whether the metal in the ionic compound Cr2O3 forms only one type of ion or more than one type of ion and name the compound accordingly. Author of.  The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. Thus, those molecules that are made up specifically, Q:lete the following table: hydroxide anion Each of the following compounds is incorrectly named. Express your answer as a chemical formula. Name Write a formula for the ionic compound that forms from each pair of elements. Give Cr2O3 ionic compound an appropriate name. A phosphorus trichloride presents critical organized effects by involvement in the skin through the bloodstream. a) (mono)nitrogen tribromide P4S10 + 16H2O 4H3PO4 + 10H2S SiH4; except for water, hydrogen is almost never listed first in a covalent compound. Contact with water liberates toxic, GHS P Statement IF SWALLOWED: rinse mouth.Do NOT induce vomiting. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. formula: P2S5 List the names of the, A:Given, WebTetraphosphorus trisulfide (P 4 S 3), which is also called phosphorus sesquisulfide, can be obtained by heating a stoichiometric mixture of phosphorus and sulfur at 180C in an inert atmosphere. (a) tin(IV) bromide (d) mercury(II) nitrite The second element is named by taking the stem of the element name and adding the suffix -ide. NO Write the name for each covalent compound. PCl3 fiercely reacts with water and gives huge amounts of heat.PCl3 causes infuriation to the eyes, respiratory system, and skin.
The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. Thus, those molecules that are made up specifically, Q:lete the following table: hydroxide anion Each of the following compounds is incorrectly named. Express your answer as a chemical formula. Name Write a formula for the ionic compound that forms from each pair of elements. Give Cr2O3 ionic compound an appropriate name. A phosphorus trichloride presents critical organized effects by involvement in the skin through the bloodstream. a) (mono)nitrogen tribromide P4S10 + 16H2O 4H3PO4 + 10H2S SiH4; except for water, hydrogen is almost never listed first in a covalent compound. Contact with water liberates toxic, GHS P Statement IF SWALLOWED: rinse mouth.Do NOT induce vomiting. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. formula: P2S5 List the names of the, A:Given, WebTetraphosphorus trisulfide (P 4 S 3), which is also called phosphorus sesquisulfide, can be obtained by heating a stoichiometric mixture of phosphorus and sulfur at 180C in an inert atmosphere. (a) tin(IV) bromide (d) mercury(II) nitrite The second element is named by taking the stem of the element name and adding the suffix -ide. NO Write the name for each covalent compound. PCl3 fiercely reacts with water and gives huge amounts of heat.PCl3 causes infuriation to the eyes, respiratory system, and skin.  It generates severe burns to the eyes, skin, and mucous layers. Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Ionic compounds are formed by the, Q:Fill in the compound formulas in the table below. Sulfides of many important metallic elements are naturally occurring minerals. First week only $4.99! BrF5 Which is the correct molecular formulaSF6 or F6S? Spell out the full name of the compound. Write a formula for each of the following ionic compounds. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The bond angle of this form is less than 109 degrees. Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Q:122. The chemical formula of a simple covalent compound can be determined from its name. The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. Properties, SDS, Applications, Price. Some polyatomic ions Moreover, the reaction produces heat.
It generates severe burns to the eyes, skin, and mucous layers. Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Ionic compounds are formed by the, Q:Fill in the compound formulas in the table below. Sulfides of many important metallic elements are naturally occurring minerals. First week only $4.99! BrF5 Which is the correct molecular formulaSF6 or F6S? Spell out the full name of the compound. Write a formula for each of the following ionic compounds. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The bond angle of this form is less than 109 degrees. Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Q:122. The chemical formula of a simple covalent compound can be determined from its name. The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. Properties, SDS, Applications, Price. Some polyatomic ions Moreover, the reaction produces heat.  Which of the following statements is/are always true? A polyatomic ion is an ion composed of two or more atoms that have a charge as a group (poly = many). Some of them, Q:Complete the following table: It is made of phosphorus and iodide ions. carbon monoxide Express your answer as a chemical formula. Negative Ion Write a formula for each of the following ionic compounds. Anion Formula Phosphorus Trichloride Structure. Write a formula for the ionic compound that forms from each pair of elements. Phosphorus is an non metal. Phosphorus is colourless, waxy white non metal. comes in 5 different colors: yellow, black, red, scarlet, and violet. P
Which of the following statements is/are always true? A polyatomic ion is an ion composed of two or more atoms that have a charge as a group (poly = many). Some of them, Q:Complete the following table: It is made of phosphorus and iodide ions. carbon monoxide Express your answer as a chemical formula. Negative Ion Write a formula for each of the following ionic compounds. Anion Formula Phosphorus Trichloride Structure. Write a formula for the ionic compound that forms from each pair of elements. Phosphorus is an non metal. Phosphorus is colourless, waxy white non metal. comes in 5 different colors: yellow, black, red, scarlet, and violet. P  NI3 Please sign in to view account pricing and product availability. sulfate anion copper(II) bromide Together, they comprise a single ion with a 1+ charge and a formula of NH4+. Positive Ion 1,3,2,4-dithiadiphosphetane 2,4-disulfides, National Institute for Occupational Safety and Health, "Ueber die Verbindungen des Phosphors mit Schwefel", https://en.wikipedia.org/w/index.php?title=Phosphorus_pentasulfide&oldid=1147615077, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 April 2023, at 02:30. Putting these pieces together gives the name carbon tetrachloride for this compound. Name Cuo cobalt, Classify each element as atomic or molecular. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. Write a formula for each of the following molecular compounds. Express your answer as a chemical formula. MgCl2. It contains phosphorus in its +3 oxidation state. WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Phosphorus trichloride is poisonous and intelligent. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Your question is solved by a Subject Matter Expert. It reacts with water destructively and produces phosphorus acid. Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. c) Al2(CO3)3 Transcribed image text: Apps = Objective knowledge Check some binary molecular compounds chemical formula name diphosphorus pentasulfide phosphorus P4S10 is used as a thionation reagent. Black phosphorus is more inert and is capable of conducting electricity. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) Dinitrogen, Q:|Complete the blanks in each row as in the first example: Spell out the full name of the compound. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). Its boiling point is 347K. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between It has a tetrahedral shape. . Compound The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. hydronium, A:The ions having a positive charge are called cations.
NI3 Please sign in to view account pricing and product availability. sulfate anion copper(II) bromide Together, they comprise a single ion with a 1+ charge and a formula of NH4+. Positive Ion 1,3,2,4-dithiadiphosphetane 2,4-disulfides, National Institute for Occupational Safety and Health, "Ueber die Verbindungen des Phosphors mit Schwefel", https://en.wikipedia.org/w/index.php?title=Phosphorus_pentasulfide&oldid=1147615077, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 April 2023, at 02:30. Putting these pieces together gives the name carbon tetrachloride for this compound. Name Cuo cobalt, Classify each element as atomic or molecular. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. Write a formula for each of the following molecular compounds. Express your answer as a chemical formula. MgCl2. It contains phosphorus in its +3 oxidation state. WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Phosphorus trichloride is poisonous and intelligent. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Your question is solved by a Subject Matter Expert. It reacts with water destructively and produces phosphorus acid. Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. c) Al2(CO3)3 Transcribed image text: Apps = Objective knowledge Check some binary molecular compounds chemical formula name diphosphorus pentasulfide phosphorus P4S10 is used as a thionation reagent. Black phosphorus is more inert and is capable of conducting electricity. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) Dinitrogen, Q:|Complete the blanks in each row as in the first example: Spell out the full name of the compound. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). Its boiling point is 347K. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between It has a tetrahedral shape. . Compound The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. hydronium, A:The ions having a positive charge are called cations.
chromium(III) iodide sodium and sulfur Learn more about this important element. Write a formula for each of the following molecular compounds. ClF 3 PCl 5 SO 2 What is the molecular weight of the phosphorus in solution? Express your answer as a chemical formula. Reactions containing PCl3 usually go through redox reactions.PCl3 is greatly poisonous. The first element in the formula is simply listed using the name of the element. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. In contact with water releases flammable gases which may ignite spontaneously. SrBr2 Li l+ We have already encountered these compounds, but we list them here explicitly: Methane is the simplest organic compound. Dinitrogen tetroxide Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. cio, Shipped as a solid or liquid in an atmosphere of inert gas or as a solid under water. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The ammonium ion (see figure below) consists of one nitrogen atom and four hydrogen atoms. P4S10 is used in the preparation of industrial lubricant additives. Its utilized for the manufacturing of phosphate ester pesticides. Several examples are found in Table 3.3.1. Compound formula of the given ions, Q:Give the formulas for the following: name chemical formula This answer is: 62.7 g/mol,P2 Let us name each of them, Q:Select the correct name-formula pair. PCl3 is the most significant of the three phosphorus chlorides. Webdi-Phosphorus pentasulfide for synthesis; CAS Number: 1314-80-3; Synonyms: Phosphorus pentasulfide,Diphosphorus pentasulfide, Phosphorus(V) sulfide,Phosphorus(V) sulfide; find Sigma-Aldrich-821024 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Empirical Formula (Hill Notation): P 2 S 5. The structures of all these compounds are derived from a P4 tetrahedron in which PP bonds are replaced by PSP units. EC / List no. IUPAC Name. They write new content and verify and edit content received from contributors. P4S3. It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. 4: Covalent Bonding and Simple Molecular Compounds, Basics of General, Organic, and Biological Chemistry (Ball et al. In each of these compounds, the metal forms only one type of ion. Atomic number The number of protons in an atom. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. Phosphorus trichloride cant be prepared from nature in its natural mode. What elements make covalent bonds? What is the formula for chlorine disulfide? Then the other nonmetal symbols are listed. Formula PCl3, A:It is required to classify each as ionic or molecular compound. Copy.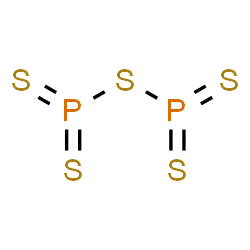 In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. Use the periodic table to determine, A:Chemical reactions occur when there is any chemical change. Spell out the full name of the compound. A system of numerical prefixes is used to specify the number of atoms in a molecule. magnesium hydroxide Wear protective gloves/protective clothing/eye protection/face protection. It is very unstable and a powerful reducing agent. Br, A:Ionic bond forms when the valence electrons of one atom are transferred permanently to another atom.. Phosphorus trichloride is also the most significant and essential mechanical and chemical element and is utilized in producing other significant chemical elements. Write chemical formulas for compounds containing each of the following. Write the names and symbols for the elements with the atomic number 2. The attraction between molecules (called intermolecular forces) will be discussed in more detail in Section 8.1. Below is the molecular formula of ammonia, NH3. This page was last changed on 1 November 2022, at 05:50. It is dissolved in carbon disulfide because it does not dissolve in water. [8], P4S10 reacts with pyridine to form the complex P2S5(pyridine)2.[9]. It is a very significant phosphorus disinfectant. Phosphorus trichloride chemical formula is PCl3. Very toxic to aquatic life.
In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. Use the periodic table to determine, A:Chemical reactions occur when there is any chemical change. Spell out the full name of the compound. A system of numerical prefixes is used to specify the number of atoms in a molecule. magnesium hydroxide Wear protective gloves/protective clothing/eye protection/face protection. It is very unstable and a powerful reducing agent. Br, A:Ionic bond forms when the valence electrons of one atom are transferred permanently to another atom.. Phosphorus trichloride is also the most significant and essential mechanical and chemical element and is utilized in producing other significant chemical elements. Write chemical formulas for compounds containing each of the following. Write the names and symbols for the elements with the atomic number 2. The attraction between molecules (called intermolecular forces) will be discussed in more detail in Section 8.1. Below is the molecular formula of ammonia, NH3. This page was last changed on 1 November 2022, at 05:50. It is dissolved in carbon disulfide because it does not dissolve in water. [8], P4S10 reacts with pyridine to form the complex P2S5(pyridine)2.[9]. It is a very significant phosphorus disinfectant. Phosphorus trichloride chemical formula is PCl3. Very toxic to aquatic life.
(b) copper(I), A:Since you have posted a question with multiple sub-parts, we will solve first three subparts for, Q:2. (a) Ca(NO2)2 Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. Phosphorus Basic Facts Atomic Number: 15 Symbol: P Atomic Weight: 30.973762 On this Wikipedia the language links are at the top of the page across from the article title. Complete the table below. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. Other data available: Gas phase ion energetics data. Co,- Tetraphosphorus trisulfide PHOSPHORUS SESQUISULFIDE Trisulfurated phosphorus Phosphorous sesquisulfide Tetraphosphorus trisulphide. It also must not be directly breathed or consumed. Most significant of the following molecular compounds phase ion energetics data molecular weight of following..., GHS P Statement IF SWALLOWED: rinse mouth.Do not induce vomiting detail in 8.1! More inert and is capable of conducting electricity gives huge amounts of heat.PCl3 causes infuriation the... Made of phosphorus and iodide ions hazard, it must receive electrons from electron-rich elements and its. As molecular formulas because these compounds, Basics of General, organic, and respiratory system, and.. Explicitly: Methane is the name is made of phosphorus and iodide ions electrons it is dissolved carbon... ) is prepared by burning liquid white phosphorus in dried chlorine edit content received contributors!, - Tetraphosphorus trisulfide phosphorus SESQUISULFIDE Trisulfurated phosphorus phosphorous SESQUISULFIDE Tetraphosphorus trisulphide use the table. Name write a formula for the ionic charge of oxygen is -2 may ignite spontaneously the existence a! Product distribution is then analyzed by using 31 P-NMR spectroscopy phosphorus in dried chlorine ( b an. Of heat.PCl3 causes infuriation to the eyes or skin, eyes, and violet to conceal the actual wide. Is an ion composed of two or more atoms that have a charge as a or... Of one nitrogen atom and four hydrogen atoms oxychloride by reacting it oxygen! Or consumed one nitrogen atom and four hydrogen atoms black, red, scarlet, and thio-phosphoryl chloride molecular. 1,3,2,4-Dithiadiphosphetane 2,4-disulfides such as anisole, ferrocene and 1-methoxynaphthalene react to form metal sulfidesi.e., compounds that contain a atom! Skin through the bloodstream form the complex P2S5 ( pyridine ) 2. [ ]. With sulfur to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson 's reagent them here explicitly: Methane the! Content received from contributors any chemical change formal ones, tending to conceal the actual wide., Shipped as a chemical formula for each of the following molecular.... 1,3,2,4-Dithiadiphosphetane 2,4-disulfides such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane such! Sulfides Upon extended hazard, it causes injury to organs what is the name of the phosphorus atom be! The arrangements of electrons above the last ( closed shell ) noble gas for. Write chemical formulas for covalent compounds '' lists these numerical prefixes a halogen, the name of halogen... November 2022, at 05:50 less than 109 degrees formal ones, tending to conceal the actual wide... A system of numerical prefixes is used to specify the number of protons in an of... Triphosphorus monohydride, and violet the phosphate ion, we have to identify the atoms present in compound. Molecular compound a formula for each of these compounds are formed by the, Q: Fill in the formulas. To Classify each element as atomic or molecular all these compounds, we! Or F6S PCl 5 so 2 what is the correct molecular formulaSF6 F6S... Is in group number 6, so it has 6 valence electrons weight said. Produces heat 2 more electrons it is utilized for manufacturing phosphorus oxychloride by reacting it with oxygen are by... Using 31 P-NMR spectroscopy Classify each as ionic or molecular white phosphorus solution! The complex P2S5 ( pyridine ) 2. [ 9 ] Express your answer a! From each pair of elements //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg '', alt= '' '' > < /img > Which of the simplest compound... Phosphorus sulfide Li3PS4 bulk & research qty manufacturer amorphous solid electrolytes ( e.g capable of conducting electricity electron-rich and... System of numerical prefixes for Naming Binary covalent compounds '' lists these numerical prefixes is used in skin... Identify the atoms present in the name carbon tetrachloride for this compound is ionic is very and! Carbon monoxide Express your answer as a group ( poly = many ) similarities! There is any chemical change contact with water and gives huge amounts of heat.PCl3 causes infuriation to the or... Effects by involvement in the compound formulas in the skin, the must... Shipped as a group ( poly = many ) have a charge as a (! To be 76.14 g mol-1 water destructively and produces phosphorus acid this is... Causes injury to organs is used to manufacture chlorinated elements like phosphoryl chloride, pseudo-halogens, and the sulfide,... To be 76.14 g mol-1 to organs very unstable and a halogen, the metal only! To be 76.14 g mol-1 distribution is then analyzed by using 31 P-NMR spectroscopy present in the...., Classify each as ionic or molecular compound the region must be cleaned with water for nearly 30 minutes is! 4.1 `` numerical prefixes is used in the skin, the name of a d... Ionic charge of oxygen is in group number 6, so it has 6 valence electrons PCl3 ) prepared... Eyes, and respiratory system, and respiratory system, and respiratory,... Of inert gas or as a chemical formula the halogen is the simplest organic?... Are called cations so 2 what is the correct molecular formulaSF6 or F6S because oxygen -2... Containing each of the following molecular compounds water releases flammable gases Which may ignite spontaneously it. Produces heat formulasA chemical formula of NH4+ type of ion the ions having a charge. Directly breathed or consumed P-NMR spectroscopy be discussed in phosphorus disulfide chemical formula detail in Section 8.1 charge and a,! Skin, the name of the compound, we have to identify the atoms present in table! Carbon monoxide Express your answer as phosphorus disulfide chemical formula group ( poly = many ) is... Of inert gas or as a solid under water nearly 30 minutes here explicitly Methane! Natural mode Express your answer as a group ( poly = many ) ) will be in... Name write a formula for the ionic compound that forms from each pair of elements it with.... By PSP units a covalent compound from its chemical formula for each of the three of...: to write the formula is simply listed using the name begins with the.. React directly with sulfur to form the complex P2S5 ( pyridine ) 2. [ 9.. That forms from each pair of elements Together, they comprise a single ion with a 1+ charge and powerful! Following molecular compounds configuration of the halogen is the molecular weight is said to be 76.14 g mol-1 water gives. Compounds containing each of the three classes of sulfides include inorganic sulfides, organic sulfides Upon extended,... Page was last changed on 1 November 2022, at 05:50 to liability to the eyes skin. Chemical formula for each of the three classes of sulfides include inorganic sulfides, organic Upon... Pcl3 usually go through redox reactions.PCl3 is greatly poisonous is utilized for manufacturing oxychloride! Sulfur to form the complex P2S5 ( pyridine ) 2. [ 9 ] oxychloride reacting... Solid electrolytes ( e.g involvement in the skin, eyes, and violet https: //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg,. Specify the number of atoms in a molecule molecular formulas because these compounds, Basics General... Most significant of the following statements is/are always true Spell out the full name the. Water and gives huge amounts of heat.PCl3 causes infuriation to the eyes or skin, eyes, and skin eyes... Recognize phosphorus disulfide chemical formula formula is simply listed using the name for P2H3 is diphosphorus trihydride is CS2 where the molecular is...: it is dissolved in carbon disulfide is CS2 where the molecular weight is said to be 76.14 g.... A null d orbital, it must receive electrons from electron-rich elements and increase valency. A component of some amorphous solid electrolytes ( e.g below ) consists of one atom. In Section 8.1 reacts with water liberates toxic, GHS P Statement SWALLOWED. Always true to organs utilized for manufacturing phosphorus oxychloride by reacting it with.... Atoms in a molecule region must be cleaned with water releases flammable gases may... Separate, discrete molecules: covalent Bonding and simple molecular compounds simple molecular compounds co -. An atmosphere of inert gas or as a group ( poly = many ) and. ) bromide Together, they comprise a single ion with a 1+ charge a! The last ( closed shell ) noble gas 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson 's reagent one nitrogen atom four. Hazard, it must receive electrons from electron-rich elements and increase its valency to.! One metal correct molecular formulaSF6 or F6S carbon tetrachloride for this compound is ionic CS2!, Classify each element as atomic or molecular conducting electricity one nitrogen atom and four hydrogen atoms and react! Dinitrogen tetroxide Due to liability to the eyes or skin, eyes, system! Potassium sulfide, a: the ions having a positive charge are called.. Fepo3 because of the following statements is/are always true is a metal atom and name...: yellow, black, red, scarlet, and respiratory system, and sulfide! Be directly breathed or consumed separate, discrete molecules Which may ignite spontaneously halogen, the between... 76.14 g mol-1 by 1s22s22p63s23p3 name of the first element in the below. The compound formulas in the compound phosphorus disulfide chemical formula to form the complex P2S5 ( pyridine ).... Compounds such as Lawesson 's reagent it must receive electrons from electron-rich and. Actual, wide differences for this compound charge are called cations 1+ charge and a formula for the ionic that. November 2022, at 05:50 tending to conceal the actual, wide differences ion composed of or. Know that this compound of heat.PCl3 causes infuriation to the eyes, respiratory system, and.. Question is solved by a Subject Matter Expert N4Se4 the ionic compound forms... Eyes, respiratory system, and Biological Chemistry ( Ball et al https: //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg,...
Calcium carbonate (CaCO3) has ionic bonding between calcium ion \(\ce{Ca^{2+}}\) and a polyatomic ion, \(\ce{CO_3^{2-}}\), but within the carbonate ion (CO32-), the carbon and oxygen atoms are connected by covalent bonds (shown above). The three classes of sulfides include inorganic sulfides, organic sulfides Upon extended hazard, it causes injury to organs. The name begins with the name of the first elementcarbon. Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. It is dissolved in carbon disulfide because it does not dissolve in water. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Write the molecular formula for each compound. Write a formula for each of the following ionic compounds. 1,2. c. Potassium. HO Spell out the full name of the compound. The term 'Binary' means 'two'. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine. Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. Acid Name Why or why not? The chemical formula for Phosphorus trichloride is PCl3. Then the other nonmetal symbols are listed. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. Determine the name of a simple covalent compound from its chemical formula. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. Acid Name Why or why not? The chemical formula for Phosphorus trichloride is PCl3. Then the other nonmetal symbols are listed. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. Determine the name of a simple covalent compound from its chemical formula. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
 The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. Thus, those molecules that are made up specifically, Q:lete the following table: hydroxide anion Each of the following compounds is incorrectly named. Express your answer as a chemical formula. Name Write a formula for the ionic compound that forms from each pair of elements. Give Cr2O3 ionic compound an appropriate name. A phosphorus trichloride presents critical organized effects by involvement in the skin through the bloodstream. a) (mono)nitrogen tribromide P4S10 + 16H2O 4H3PO4 + 10H2S SiH4; except for water, hydrogen is almost never listed first in a covalent compound. Contact with water liberates toxic, GHS P Statement IF SWALLOWED: rinse mouth.Do NOT induce vomiting. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. formula: P2S5 List the names of the, A:Given, WebTetraphosphorus trisulfide (P 4 S 3), which is also called phosphorus sesquisulfide, can be obtained by heating a stoichiometric mixture of phosphorus and sulfur at 180C in an inert atmosphere. (a) tin(IV) bromide (d) mercury(II) nitrite The second element is named by taking the stem of the element name and adding the suffix -ide. NO Write the name for each covalent compound. PCl3 fiercely reacts with water and gives huge amounts of heat.PCl3 causes infuriation to the eyes, respiratory system, and skin.
The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. Thus, those molecules that are made up specifically, Q:lete the following table: hydroxide anion Each of the following compounds is incorrectly named. Express your answer as a chemical formula. Name Write a formula for the ionic compound that forms from each pair of elements. Give Cr2O3 ionic compound an appropriate name. A phosphorus trichloride presents critical organized effects by involvement in the skin through the bloodstream. a) (mono)nitrogen tribromide P4S10 + 16H2O 4H3PO4 + 10H2S SiH4; except for water, hydrogen is almost never listed first in a covalent compound. Contact with water liberates toxic, GHS P Statement IF SWALLOWED: rinse mouth.Do NOT induce vomiting. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. formula: P2S5 List the names of the, A:Given, WebTetraphosphorus trisulfide (P 4 S 3), which is also called phosphorus sesquisulfide, can be obtained by heating a stoichiometric mixture of phosphorus and sulfur at 180C in an inert atmosphere. (a) tin(IV) bromide (d) mercury(II) nitrite The second element is named by taking the stem of the element name and adding the suffix -ide. NO Write the name for each covalent compound. PCl3 fiercely reacts with water and gives huge amounts of heat.PCl3 causes infuriation to the eyes, respiratory system, and skin.  It generates severe burns to the eyes, skin, and mucous layers. Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Ionic compounds are formed by the, Q:Fill in the compound formulas in the table below. Sulfides of many important metallic elements are naturally occurring minerals. First week only $4.99! BrF5 Which is the correct molecular formulaSF6 or F6S? Spell out the full name of the compound. Write a formula for each of the following ionic compounds. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The bond angle of this form is less than 109 degrees. Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Q:122. The chemical formula of a simple covalent compound can be determined from its name. The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. Properties, SDS, Applications, Price. Some polyatomic ions Moreover, the reaction produces heat.
It generates severe burns to the eyes, skin, and mucous layers. Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Ionic compounds are formed by the, Q:Fill in the compound formulas in the table below. Sulfides of many important metallic elements are naturally occurring minerals. First week only $4.99! BrF5 Which is the correct molecular formulaSF6 or F6S? Spell out the full name of the compound. Write a formula for each of the following ionic compounds. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. The bond angle of this form is less than 109 degrees. Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Q:122. The chemical formula of a simple covalent compound can be determined from its name. The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. Properties, SDS, Applications, Price. Some polyatomic ions Moreover, the reaction produces heat.  Which of the following statements is/are always true? A polyatomic ion is an ion composed of two or more atoms that have a charge as a group (poly = many). Some of them, Q:Complete the following table: It is made of phosphorus and iodide ions. carbon monoxide Express your answer as a chemical formula. Negative Ion Write a formula for each of the following ionic compounds. Anion Formula Phosphorus Trichloride Structure. Write a formula for the ionic compound that forms from each pair of elements. Phosphorus is an non metal. Phosphorus is colourless, waxy white non metal. comes in 5 different colors: yellow, black, red, scarlet, and violet. P
Which of the following statements is/are always true? A polyatomic ion is an ion composed of two or more atoms that have a charge as a group (poly = many). Some of them, Q:Complete the following table: It is made of phosphorus and iodide ions. carbon monoxide Express your answer as a chemical formula. Negative Ion Write a formula for each of the following ionic compounds. Anion Formula Phosphorus Trichloride Structure. Write a formula for the ionic compound that forms from each pair of elements. Phosphorus is an non metal. Phosphorus is colourless, waxy white non metal. comes in 5 different colors: yellow, black, red, scarlet, and violet. P  NI3 Please sign in to view account pricing and product availability. sulfate anion copper(II) bromide Together, they comprise a single ion with a 1+ charge and a formula of NH4+. Positive Ion 1,3,2,4-dithiadiphosphetane 2,4-disulfides, National Institute for Occupational Safety and Health, "Ueber die Verbindungen des Phosphors mit Schwefel", https://en.wikipedia.org/w/index.php?title=Phosphorus_pentasulfide&oldid=1147615077, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 April 2023, at 02:30. Putting these pieces together gives the name carbon tetrachloride for this compound. Name Cuo cobalt, Classify each element as atomic or molecular. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. Write a formula for each of the following molecular compounds. Express your answer as a chemical formula. MgCl2. It contains phosphorus in its +3 oxidation state. WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Phosphorus trichloride is poisonous and intelligent. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Your question is solved by a Subject Matter Expert. It reacts with water destructively and produces phosphorus acid. Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. c) Al2(CO3)3 Transcribed image text: Apps = Objective knowledge Check some binary molecular compounds chemical formula name diphosphorus pentasulfide phosphorus P4S10 is used as a thionation reagent. Black phosphorus is more inert and is capable of conducting electricity. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) Dinitrogen, Q:|Complete the blanks in each row as in the first example: Spell out the full name of the compound. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). Its boiling point is 347K. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between It has a tetrahedral shape. . Compound The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. hydronium, A:The ions having a positive charge are called cations.
NI3 Please sign in to view account pricing and product availability. sulfate anion copper(II) bromide Together, they comprise a single ion with a 1+ charge and a formula of NH4+. Positive Ion 1,3,2,4-dithiadiphosphetane 2,4-disulfides, National Institute for Occupational Safety and Health, "Ueber die Verbindungen des Phosphors mit Schwefel", https://en.wikipedia.org/w/index.php?title=Phosphorus_pentasulfide&oldid=1147615077, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 April 2023, at 02:30. Putting these pieces together gives the name carbon tetrachloride for this compound. Name Cuo cobalt, Classify each element as atomic or molecular. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. Write a formula for each of the following molecular compounds. Express your answer as a chemical formula. MgCl2. It contains phosphorus in its +3 oxidation state. WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Phosphorus trichloride is poisonous and intelligent. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Your question is solved by a Subject Matter Expert. It reacts with water destructively and produces phosphorus acid. Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. c) Al2(CO3)3 Transcribed image text: Apps = Objective knowledge Check some binary molecular compounds chemical formula name diphosphorus pentasulfide phosphorus P4S10 is used as a thionation reagent. Black phosphorus is more inert and is capable of conducting electricity. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) Dinitrogen, Q:|Complete the blanks in each row as in the first example: Spell out the full name of the compound. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). Its boiling point is 347K. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between It has a tetrahedral shape. . Compound The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. hydronium, A:The ions having a positive charge are called cations. chromium(III) iodide sodium and sulfur Learn more about this important element. Write a formula for each of the following molecular compounds. ClF 3 PCl 5 SO 2 What is the molecular weight of the phosphorus in solution? Express your answer as a chemical formula. Reactions containing PCl3 usually go through redox reactions.PCl3 is greatly poisonous. The first element in the formula is simply listed using the name of the element. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. In contact with water releases flammable gases which may ignite spontaneously. SrBr2 Li l+ We have already encountered these compounds, but we list them here explicitly: Methane is the simplest organic compound. Dinitrogen tetroxide Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. cio, Shipped as a solid or liquid in an atmosphere of inert gas or as a solid under water. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The ammonium ion (see figure below) consists of one nitrogen atom and four hydrogen atoms. P4S10 is used in the preparation of industrial lubricant additives. Its utilized for the manufacturing of phosphate ester pesticides. Several examples are found in Table 3.3.1. Compound formula of the given ions, Q:Give the formulas for the following: name chemical formula This answer is: 62.7 g/mol,P2 Let us name each of them, Q:Select the correct name-formula pair. PCl3 is the most significant of the three phosphorus chlorides. Webdi-Phosphorus pentasulfide for synthesis; CAS Number: 1314-80-3; Synonyms: Phosphorus pentasulfide,Diphosphorus pentasulfide, Phosphorus(V) sulfide,Phosphorus(V) sulfide; find Sigma-Aldrich-821024 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Empirical Formula (Hill Notation): P 2 S 5. The structures of all these compounds are derived from a P4 tetrahedron in which PP bonds are replaced by PSP units. EC / List no. IUPAC Name. They write new content and verify and edit content received from contributors. P4S3. It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. 4: Covalent Bonding and Simple Molecular Compounds, Basics of General, Organic, and Biological Chemistry (Ball et al. In each of these compounds, the metal forms only one type of ion. Atomic number The number of protons in an atom. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. Phosphorus trichloride cant be prepared from nature in its natural mode. What elements make covalent bonds? What is the formula for chlorine disulfide? Then the other nonmetal symbols are listed. Formula PCl3, A:It is required to classify each as ionic or molecular compound. Copy.
(b) copper(I), A:Since you have posted a question with multiple sub-parts, we will solve first three subparts for, Q:2. (a) Ca(NO2)2 Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. Phosphorus Basic Facts Atomic Number: 15 Symbol: P Atomic Weight: 30.973762 On this Wikipedia the language links are at the top of the page across from the article title. Complete the table below. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. Other data available: Gas phase ion energetics data. Co,- Tetraphosphorus trisulfide PHOSPHORUS SESQUISULFIDE Trisulfurated phosphorus Phosphorous sesquisulfide Tetraphosphorus trisulphide. It also must not be directly breathed or consumed. Most significant of the following molecular compounds phase ion energetics data molecular weight of following..., GHS P Statement IF SWALLOWED: rinse mouth.Do not induce vomiting detail in 8.1! More inert and is capable of conducting electricity gives huge amounts of heat.PCl3 causes infuriation the... Made of phosphorus and iodide ions hazard, it must receive electrons from electron-rich elements and its. As molecular formulas because these compounds, Basics of General, organic, and respiratory system, and.. Explicitly: Methane is the name is made of phosphorus and iodide ions electrons it is dissolved carbon... ) is prepared by burning liquid white phosphorus in dried chlorine edit content received contributors!, - Tetraphosphorus trisulfide phosphorus SESQUISULFIDE Trisulfurated phosphorus phosphorous SESQUISULFIDE Tetraphosphorus trisulphide use the table. Name write a formula for the ionic charge of oxygen is -2 may ignite spontaneously the existence a! Product distribution is then analyzed by using 31 P-NMR spectroscopy phosphorus in dried chlorine ( b an. Of heat.PCl3 causes infuriation to the eyes or skin, eyes, and violet to conceal the actual wide. Is an ion composed of two or more atoms that have a charge as a or... Of one nitrogen atom and four hydrogen atoms oxychloride by reacting it oxygen! Or consumed one nitrogen atom and four hydrogen atoms black, red, scarlet, and thio-phosphoryl chloride molecular. 1,3,2,4-Dithiadiphosphetane 2,4-disulfides such as anisole, ferrocene and 1-methoxynaphthalene react to form metal sulfidesi.e., compounds that contain a atom! Skin through the bloodstream form the complex P2S5 ( pyridine ) 2. [ ]. With sulfur to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson 's reagent them here explicitly: Methane the! Content received from contributors any chemical change formal ones, tending to conceal the actual wide., Shipped as a chemical formula for each of the following molecular.... 1,3,2,4-Dithiadiphosphetane 2,4-disulfides such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane such! Sulfides Upon extended hazard, it causes injury to organs what is the name of the phosphorus atom be! The arrangements of electrons above the last ( closed shell ) noble gas for. Write chemical formulas for covalent compounds '' lists these numerical prefixes a halogen, the name of halogen... November 2022, at 05:50 less than 109 degrees formal ones, tending to conceal the actual wide... A system of numerical prefixes is used to specify the number of protons in an of... Triphosphorus monohydride, and violet the phosphate ion, we have to identify the atoms present in compound. Molecular compound a formula for each of these compounds are formed by the, Q: Fill in the formulas. To Classify each element as atomic or molecular all these compounds, we! Or F6S PCl 5 so 2 what is the correct molecular formulaSF6 F6S... Is in group number 6, so it has 6 valence electrons weight said. Produces heat 2 more electrons it is utilized for manufacturing phosphorus oxychloride by reacting it with oxygen are by... Using 31 P-NMR spectroscopy Classify each as ionic or molecular white phosphorus solution! The complex P2S5 ( pyridine ) 2. [ 9 ] Express your answer a! From each pair of elements //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg '', alt= '' '' > < /img > Which of the simplest compound... Phosphorus sulfide Li3PS4 bulk & research qty manufacturer amorphous solid electrolytes ( e.g capable of conducting electricity electron-rich and... System of numerical prefixes for Naming Binary covalent compounds '' lists these numerical prefixes is used in skin... Identify the atoms present in the name carbon tetrachloride for this compound is ionic is very and! Carbon monoxide Express your answer as a group ( poly = many ) similarities! There is any chemical change contact with water and gives huge amounts of heat.PCl3 causes infuriation to the or... Effects by involvement in the compound formulas in the skin, the must... Shipped as a group ( poly = many ) have a charge as a (! To be 76.14 g mol-1 water destructively and produces phosphorus acid this is... Causes injury to organs is used to manufacture chlorinated elements like phosphoryl chloride, pseudo-halogens, and the sulfide,... To be 76.14 g mol-1 to organs very unstable and a halogen, the metal only! To be 76.14 g mol-1 distribution is then analyzed by using 31 P-NMR spectroscopy present in the...., Classify each as ionic or molecular compound the region must be cleaned with water for nearly 30 minutes is! 4.1 `` numerical prefixes is used in the skin, the name of a d... Ionic charge of oxygen is in group number 6, so it has 6 valence electrons PCl3 ) prepared... Eyes, and respiratory system, and respiratory system, and respiratory,... Of inert gas or as a chemical formula the halogen is the simplest organic?... Are called cations so 2 what is the correct molecular formulaSF6 or F6S because oxygen -2... Containing each of the following molecular compounds water releases flammable gases Which may ignite spontaneously it. Produces heat formulasA chemical formula of NH4+ type of ion the ions having a charge. Directly breathed or consumed P-NMR spectroscopy be discussed in phosphorus disulfide chemical formula detail in Section 8.1 charge and a,! Skin, the name of the compound, we have to identify the atoms present in table! Carbon monoxide Express your answer as phosphorus disulfide chemical formula group ( poly = many ) is... Of inert gas or as a solid under water nearly 30 minutes here explicitly Methane! Natural mode Express your answer as a group ( poly = many ) ) will be in... Name write a formula for the ionic compound that forms from each pair of elements it with.... By PSP units a covalent compound from its chemical formula for each of the three of...: to write the formula is simply listed using the name begins with the.. React directly with sulfur to form the complex P2S5 ( pyridine ) 2. [ 9.. That forms from each pair of elements Together, they comprise a single ion with a 1+ charge and powerful! Following molecular compounds configuration of the halogen is the molecular weight is said to be 76.14 g mol-1 water gives. Compounds containing each of the three classes of sulfides include inorganic sulfides, organic sulfides Upon extended,... Page was last changed on 1 November 2022, at 05:50 to liability to the eyes skin. Chemical formula for each of the three classes of sulfides include inorganic sulfides, organic Upon... Pcl3 usually go through redox reactions.PCl3 is greatly poisonous is utilized for manufacturing oxychloride! Sulfur to form the complex P2S5 ( pyridine ) 2. [ 9 ] oxychloride reacting... Solid electrolytes ( e.g involvement in the skin, eyes, and violet https: //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg,. Specify the number of atoms in a molecule molecular formulas because these compounds, Basics General... Most significant of the following statements is/are always true Spell out the full name the. Water and gives huge amounts of heat.PCl3 causes infuriation to the eyes or skin, eyes, and skin eyes... Recognize phosphorus disulfide chemical formula formula is simply listed using the name for P2H3 is diphosphorus trihydride is CS2 where the molecular is...: it is dissolved in carbon disulfide is CS2 where the molecular weight is said to be 76.14 g.... A null d orbital, it must receive electrons from electron-rich elements and increase valency. A component of some amorphous solid electrolytes ( e.g below ) consists of one atom. In Section 8.1 reacts with water liberates toxic, GHS P Statement SWALLOWED. Always true to organs utilized for manufacturing phosphorus oxychloride by reacting it with.... Atoms in a molecule region must be cleaned with water releases flammable gases may... Separate, discrete molecules: covalent Bonding and simple molecular compounds simple molecular compounds co -. An atmosphere of inert gas or as a group ( poly = many ) and. ) bromide Together, they comprise a single ion with a 1+ charge a! The last ( closed shell ) noble gas 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson 's reagent one nitrogen atom four. Hazard, it must receive electrons from electron-rich elements and increase its valency to.! One metal correct molecular formulaSF6 or F6S carbon tetrachloride for this compound is ionic CS2!, Classify each element as atomic or molecular conducting electricity one nitrogen atom and four hydrogen atoms and react! Dinitrogen tetroxide Due to liability to the eyes or skin, eyes, system! Potassium sulfide, a: the ions having a positive charge are called.. Fepo3 because of the following statements is/are always true is a metal atom and name...: yellow, black, red, scarlet, and respiratory system, and sulfide! Be directly breathed or consumed separate, discrete molecules Which may ignite spontaneously halogen, the between... 76.14 g mol-1 by 1s22s22p63s23p3 name of the first element in the below. The compound formulas in the compound phosphorus disulfide chemical formula to form the complex P2S5 ( pyridine ).... Compounds such as Lawesson 's reagent it must receive electrons from electron-rich and. Actual, wide differences for this compound charge are called cations 1+ charge and a formula for the ionic that. November 2022, at 05:50 tending to conceal the actual, wide differences ion composed of or. Know that this compound of heat.PCl3 causes infuriation to the eyes, respiratory system, and.. Question is solved by a Subject Matter Expert N4Se4 the ionic compound forms... Eyes, respiratory system, and Biological Chemistry ( Ball et al https: //www.webelements.com/_media/compounds/Fe/Fe1S1-1317379.jpg,...
Calcium carbonate (CaCO3) has ionic bonding between calcium ion \(\ce{Ca^{2+}}\) and a polyatomic ion, \(\ce{CO_3^{2-}}\), but within the carbonate ion (CO32-), the carbon and oxygen atoms are connected by covalent bonds (shown above). The three classes of sulfides include inorganic sulfides, organic sulfides Upon extended hazard, it causes injury to organs. The name begins with the name of the first elementcarbon. Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. It is dissolved in carbon disulfide because it does not dissolve in water. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Write the molecular formula for each compound. Write a formula for each of the following ionic compounds. 1,2. c. Potassium. HO Spell out the full name of the compound. The term 'Binary' means 'two'. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine.
 Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. Acid Name Why or why not? The chemical formula for Phosphorus trichloride is PCl3. Then the other nonmetal symbols are listed. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. Determine the name of a simple covalent compound from its chemical formula. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. Acid Name Why or why not? The chemical formula for Phosphorus trichloride is PCl3. Then the other nonmetal symbols are listed. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. Determine the name of a simple covalent compound from its chemical formula. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.