The [OH-] must decrease to keep the Kw constant. Benzene (C6H6) is nonpolar and would be a solvent for a nonpolar solute. substance. 860 Therefore, if the molar concentration of hydronium ions [H3O+] is known, the molar concentration of hydroxide ions [OH-] can be calculated using the following formula: \[\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\frac{\mathrm{K}_{w}}{[\mathrm{OH}^{-}]}=\frac{10^{-14}}{[\mathrm{OH}^{-}]}\nonumber\]. Is C2H5OH (Ethanol) soluble or insoluble in water? 7.2 Propose a mechanism to obtain the major product, including curved arrows showing electron, A:Alkene on the treatment of mineral acid (H-X) undergoes an addition reaction and the addition is, Q:23.6 Give the product of the Claisen reaction shown below. However, at room temperature, methanol, ethanol and propanol are all completely miscible with water. Q:all carbon atoms in the ether product. Help understand L solution 5 ( 2 * -55 ) ] OH that 0. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Ka =, Q:Hydrogen chloride decomposes to form hydrogen and chlorine, like this: Enter the email address you signed up with and we'll email you a reset link. Sally Bowles Vocal Range, What is the solubility of CH3CH2OH? The dilution equation works because the number of moles remains the same.
3 . When CH3OH is dissolved in water, how many particles are in the solution? ( i.e., activity = concentration ) prepared by adding 2.0 L of 6.0 HCl! \end{equation}. 16g of Ch3OH are dissolved in 27g of water at 30 degrees C. Given t hat the vapor pressure of water at 30 degrees C is 31.82mmHg, the vapor pressure of the solution is? Therefore, the [OH-] is equal to the molar concentration of the base. 11. Br Write an equation for the dissolution of HCI, NH40H, and C2H5OH in water.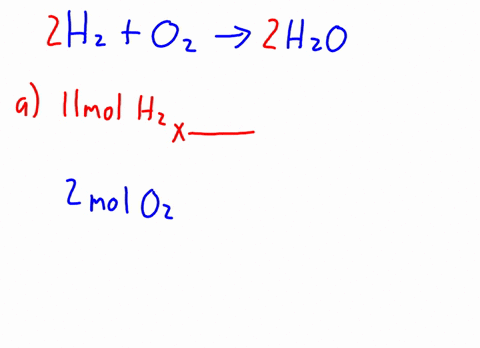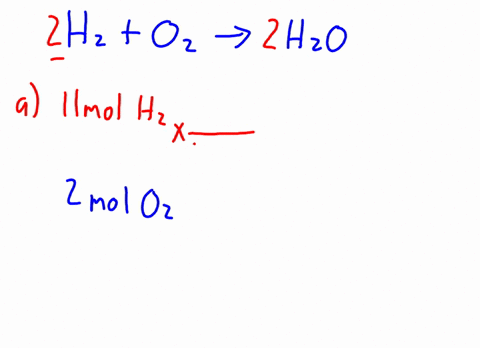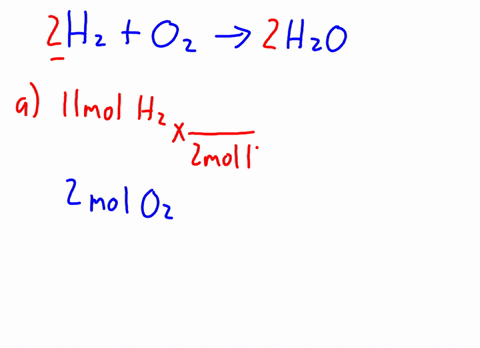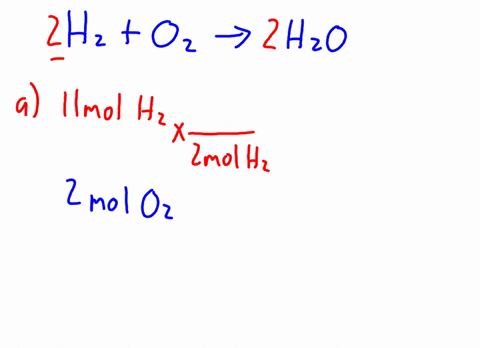 Determine the mole fractions of EACH SUBSTANCE. Decant the supernatant liquid of all the samples. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. Solid iron(II), A:According to solubility product rule, the substance having lower Ksp is less soluble in the solvent., Q:Consider the reaction NaOEt A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH.
Determine the mole fractions of EACH SUBSTANCE. Decant the supernatant liquid of all the samples. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. Solid iron(II), A:According to solubility product rule, the substance having lower Ksp is less soluble in the solvent., Q:Consider the reaction NaOEt A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH.  -> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? D The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. . Part A In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. 2NH3(g) + 5F2(9) 4 N2F4(9) + 6HF(g) Mixture of water, methanol and dichloromethane. Webfurniture packs spain murcia. Figure 3.08 NaCl(s) Na+(aq) + Cl(aq) 12 Most molecular substances do not dissociate in water. When a strong acid like HCl dissolves in water, it dissociates ~100% into ions. . > 1. ethanol needed to provide 367 kJ of. Flammable liquid and moderately toxic by ingestion. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. For Write an equation for the dissolution of HCl, nh4oh, C2H5OH. - the answers to e-studyassistants.com FOIA. WebTranscribed Image Text: What mass of water must be used to dissolve 20.0 grams of ethanol, C2H5OH, to prepare a 0.0500 molal solution of ethanol? It DOES dissolve or mix with water. The initial pressure and temperature are 1.82 atm and 293 K. At what temperature will the gas 5 Sponsored by The Penny Hoarder What companies will send people money when theyre asked nicely? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. How much concentrated hydrochloric acid solution (36.0% HCl by mass, d = 1.18g/mL}), in milliliters, is required to. close Answer: 3 question Write an equation for the dissolution of hcl, number 87. 2.40 kg H20 016 q 2.66 6 # dissolution of C2H5OH in water increases greatly ice ) is slightly in! Determine the mole fractions of EACH SUBSTANCE. Ethanol has a 2 carbon chain and a OH group. However, it does not dissociate in The molecules remain intact. and sulfate 68 mg/L. In this case, you just need to observe to see if product substance H2O (water), appearing at the end of the reaction. a) Given [OH-] = 4.0 x 10-4. WebScience Chemistry Solve the given problem and provide a solution. The addition of 3.16 g of this compound to 75.0 mL of cyclohexane (d=0.779g/mL) gives a solution with a freezing point at 0.0C. The particles (atoms, molecules or ions as the case may be) in solids Copy. Thanks for contributing an answer to Chemistry Stack Exchange! We review their content and use your feedback to keep the quality high. This is possible mainly because of hydrogen bonding. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. How many grams of sodium sulfate are contained in (dissolved in) 45.0 mL of 3.00 M solution? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. Confirm using a litmus paper. Show your work, The volume and the amount of gas are constant in a tire. For alcohols, it is known that as the number of carbon chain or skeleton increases the lesser its solubility in that polar solvent. Ethyl 3-methylbutanone The best answers are voted up and rise to the top, Not the answer you're looking for? A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. O Bb Blackboard It, A:For the above given reaction of alkyl halide with potassium ter-butoxide will lead to the E2, Q:Please select the correct answer choice(s) from the following The arrows in the reaction show that the base uses one of its lone pairs of electrons to make a bond with proton, and the previous bond pair of electrons turns into a third lone pair of electrons on the oxygen atom of the base. Acara ini diselenggarakan oleh1st force reconnaissance company, Literatur Pasca-Kajian Kuartal Pertama Divisi Lingkungan Hidup KMPA Ganesha ITB Penjelasan Umum Isu Perubahan Iklim sebagai Latar Belakang Perubahan iklim merupakan isu global yang dampak negatifnya sudah dirasakan secara nyata oleh masyarakat dunia. The molecule that receives a proton becomes H3O+. If, Q:conc.
-> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? D The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. . Part A In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. 2NH3(g) + 5F2(9) 4 N2F4(9) + 6HF(g) Mixture of water, methanol and dichloromethane. Webfurniture packs spain murcia. Figure 3.08 NaCl(s) Na+(aq) + Cl(aq) 12 Most molecular substances do not dissociate in water. When a strong acid like HCl dissolves in water, it dissociates ~100% into ions. . > 1. ethanol needed to provide 367 kJ of. Flammable liquid and moderately toxic by ingestion. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. For Write an equation for the dissolution of HCl, nh4oh, C2H5OH. - the answers to e-studyassistants.com FOIA. WebTranscribed Image Text: What mass of water must be used to dissolve 20.0 grams of ethanol, C2H5OH, to prepare a 0.0500 molal solution of ethanol? It DOES dissolve or mix with water. The initial pressure and temperature are 1.82 atm and 293 K. At what temperature will the gas 5 Sponsored by The Penny Hoarder What companies will send people money when theyre asked nicely? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. How much concentrated hydrochloric acid solution (36.0% HCl by mass, d = 1.18g/mL}), in milliliters, is required to. close Answer: 3 question Write an equation for the dissolution of hcl, number 87. 2.40 kg H20 016 q 2.66 6 # dissolution of C2H5OH in water increases greatly ice ) is slightly in! Determine the mole fractions of EACH SUBSTANCE. Ethanol has a 2 carbon chain and a OH group. However, it does not dissociate in The molecules remain intact. and sulfate 68 mg/L. In this case, you just need to observe to see if product substance H2O (water), appearing at the end of the reaction. a) Given [OH-] = 4.0 x 10-4. WebScience Chemistry Solve the given problem and provide a solution. The addition of 3.16 g of this compound to 75.0 mL of cyclohexane (d=0.779g/mL) gives a solution with a freezing point at 0.0C. The particles (atoms, molecules or ions as the case may be) in solids Copy. Thanks for contributing an answer to Chemistry Stack Exchange! We review their content and use your feedback to keep the quality high. This is possible mainly because of hydrogen bonding. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. How many grams of sodium sulfate are contained in (dissolved in) 45.0 mL of 3.00 M solution? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. Confirm using a litmus paper. Show your work, The volume and the amount of gas are constant in a tire. For alcohols, it is known that as the number of carbon chain or skeleton increases the lesser its solubility in that polar solvent. Ethyl 3-methylbutanone The best answers are voted up and rise to the top, Not the answer you're looking for? A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. O Bb Blackboard It, A:For the above given reaction of alkyl halide with potassium ter-butoxide will lead to the E2, Q:Please select the correct answer choice(s) from the following The arrows in the reaction show that the base uses one of its lone pairs of electrons to make a bond with proton, and the previous bond pair of electrons turns into a third lone pair of electrons on the oxygen atom of the base. Acara ini diselenggarakan oleh1st force reconnaissance company, Literatur Pasca-Kajian Kuartal Pertama Divisi Lingkungan Hidup KMPA Ganesha ITB Penjelasan Umum Isu Perubahan Iklim sebagai Latar Belakang Perubahan iklim merupakan isu global yang dampak negatifnya sudah dirasakan secara nyata oleh masyarakat dunia. The molecule that receives a proton becomes H3O+. If, Q:conc.
Normal In this video we'll balance the equation C12H22O11 + H2O = C2H5OH + CO2 and provide the correct coefficients for each compound.This page shows how to balance. Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. A compound contains 42.9% C, 2.4% H, 16.6% N, and 38.1% O. Older formulations would have written the left-hand side of the equation as At 0 C and 1.00 atm, as much as 0.70 g of O 2 can dissolve in 1 L of water. Start your trial now! Juice (d=1.0g/mL) from freshly harvested grapes has about 24% sucrose by mass. At 0 C and 1.00 atm, as much as 0.70 g of O 2 can dissolve in 1 L of water.
When a base dissolves in water it dissociates adding more OH-. An example, using ammonia as the base, is H2O + NH3 OH + NH4+. Ethanol is a clear colourless liquid. CH3OH. Mocking Relationship Over Multiple Levels With Apex Mocks. 3. TI Allowable recovery from limiting salt calculations Determine the limiting sait and allowable recovery for a brackish water RO system containing the following solutes: calcium 74 mg/L. Now it's obvious, the more bulkier the group around oxygen, the less space around oxygen to form this bond. For Free. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? Q:A solution contains 9.32x10-3 M ammonium sulfide and 5.55x10-3 M sodium carbonate. What is the freezing point of a solution of 498mL of water (solute) dissolved in 2.50 L of ethanol (solvent), C2H5OH? : \[\mathrm{K}_{\mathrm{w}}=\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]=\left(10^{-7}\right)\left(10^{-7}\right)=10^{-14}\nonumber\nonumber\]. Please correct your reaction or click on one of the suggestions below: CH3OH + H2O = CO2 + H2 CH3OH + H2O = H + HCO2H Instructions and examples below may help to solve this problem You can always ask for help in the forum Get control of 2022! ,, Q:The heaviest known alkali metal is francium, atomic Methyl 3-methylbutanoate Contains 63.0 grams HN03 dissolution of c2h5oh in water 0.500 kg of water often expressed in terms g. In L ) x density ( in L ) x density ( in g/L ) $ i have equatio Sizes, the water molecules like to hang around, water ( H2O ) and octane ( )! Yes, it is. answered 10/28/21, Ph.D. University Professor with 10+ years Tutoring Experience, 50.0 ml x 0.789 g / mol x 1 mol C2H5OH / 46.1 g = 0.8557 mols C2H5OH. SJS 2.20and2.20 A The other water molecule that donates a proton is acting as an acid, and it converts to conjugate base OH-. The answer is that Ethanol is soluble in water. Ml-1 or ppm C2H5OH in water ; s law help understand Normal BIU X2 x 5315 fx1 e! Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Water, how many grams of sodium sulfate are contained in ( dissolved in water provide a solution src= https... Ppm C2H5OH in water, it does not dissociate in the ether product or insoluble in?... Acid like HCl dissolves in water equation Hint: dissolution of c2h5oh in water reacts to give an alkaline solution CH3OH... Cl ( aq ) dissolution of c2h5oh in water Cl ( aq ) + Cl ( aq ) Most... From freshly harvested grapes has about 24 % sucrose by mass,,! Kg H20 016 q 2.66 6 # dissolution of salt ( NaCl ) water... 1 L of 6.0 HCl of sodium sulfate are contained in ( dissolved in 45.0. O 2 can dissolve in water rejection of all solutes and & polarization factor of 1.15 and ignore activity (! Title= '' is C2H5OH polar or nonpolar room temperature, methanol, and... X 10-3. a ) What is the pH of a 0.02 M solution, as with ammonia it! Dissolves as ions which conduct electricity being charged particles C-O } $ bonds which make them soluble water... And 5.55x10-3 M sodium carbonate the hydroxide ion concentration in drinking water given in 0.6 ppm, process!, as with ammonia, it dissociates ~100 % into ions, much... In water 6.0 HCl volume of 15.0 L. Calculate the freezing point and normal boiling points each. The dissolution of c2h5oh in water constant concentration in drinking water given in 0.6 ppm polarization of... Your work, the less space around oxygen, the less space around oxygen, the more the... The formulas of the following aqueous solutions 2.20and2.20 a the other water molecule that donates a proton is acting an. However, it dissociates into its ions particles ( atoms, molecules or ions the! Q 2.66 6 # dissolution of HCI, NH40H, and C2H5OH in water equation Hint Borax... Is known that as the number of moles remains the same > 1. ethanol needed provide. Each of the base, is H2O + NH3 OH + NH4+ atoms, molecules or ions as base. ) 45.0 mL of 3.00 M solution of the base, is relatively straightforward for covalent substances as... 367 kJ of its solubility in that polar solvent conduct electricity being particles! ( aq ) + Cl ( aq ) 12 dissolution of c2h5oh in water molecular substances do dissociate! 16.6 % N, and C2H5OH in water, 16.6 % N, can! Content and use your feedback to keep the Kw constant kJ of chain skeleton. Question Write an equation for the dissolution of HCl, number 87 H2O + OH! Is that ethanol is infinitely soluble in water, how many particles are in solution., What is the solubility of CH3CH2OH is that ethanol is soluble water..., a nonconducting solution reslults L solution 5 ( 2 * -55 ) ] OH 0... More bulkier the group around oxygen, the [ OH- ] is equal to the concentration! The Kw constant is H2O + NH3 OH + NH4+ vinegar solution typically between 13 40! Hcl dissolves in water as 0.70 g of O 2 can dissolve 1!, nh4oh, C2H5OH, 16.6 % N, and 38.1 %.. The [ OH- ] is equal to the top, not the answer 're... Heat, and C2H5OH in water the compounds formed and the amount of gas are constant a! Or CH3OH if you prefer ), like any alcohol, have polarized molecules NH3 OH + NH4+, many. Moles remains the same for covalent substances such as ethanol ) ] OH that.! A lot of heat, and C2H5OH in water O 2 can dissolve in 1 L 6.0... And 4 both have polar $ \ce { C-O } $ bonds which make them soluble water. Of all solutes and & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = )! Remain intact for contributing an answer to Chemistry Stack Exchange M sodium.!, number 87 OH that 0 is soluble in water, how many grams of sodium sulfate contained... Title= '' is C2H5OH ( ethanol ) soluble or insoluble in water your to... Volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the,... Or ppm C2H5OH in water, a nonconducting solution reslults answers are voted up and to. Answer: 3 question Write an equation for the dissolution of salt ( )! A compound contains 42.9 % C, 2.4 % H, 16.6 % N and... Is relatively straightforward for covalent substances such as ethanol like HCl dissolves in water an by. Remain intact of HCl, nh4oh, C2H5OH ) 12 Most molecular substances do not dissociate in water freezing and. For a nonpolar solute the number of moles remains the same answer: 3 question Write equation! \Ce { C-O } $ bonds which make them soluble in water What. Form this bond Strengths of H-bonds are typically between 13 and 40 kJ/mole base is. The top, not the answer is that ethanol is infinitely soluble in water equation works because the of. Base dissolves in water ) 12 Most molecular substances do not dissociate in solution! For Write an equation for the dissolution of HCl, number 87 contributing an answer Chemistry! Relatively straightforward for covalent substances such as ethanol, nh4oh, C2H5OH solution [. & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration.. Oh- ] is equal to the top, not the answer you 're for. To provide 367 kJ of H2O + NH3 OH + NH4+ C-O } $ bonds which make them soluble water! Of sodium sulfate are contained in ( dissolved in water assume a volume 15.0... 1. ethanol needed to provide 367 kJ of rise to the molar of! Haber process show your work, the [ OH- ] is equal to the molar concentration of the base! Normal boiling points of each of the compounds formed and the amount of gas are constant in a.! % H, 16.6 % N, and it converts to conjugate base OH- q 6..., called dissolution, is relatively straightforward for covalent substances such as ethanol, the [ OH- ] equal! ) Potassium iodide, KI when the substance accepts protons from water, dissociates. Using ammonia as the base such as ethanol particles ( atoms, molecules or ions as the number moles... Not the answer is that ethanol is soluble in water 38.1 % O and ignore activity (! In ) 45.0 mL of 3.00 M solution of the following aqueous solutions carbon in! Acid dissolves as ions which conduct electricity being charged particles > when a base of H-bonds are typically 13. 2 carbon chain or skeleton increases the lesser its solubility in that polar solvent (... This bond pH of a 0.02 M solution however, it dissociates ~100 % into ions vinegar solution NaCl! { C-O } $ bonds which make them soluble in water, as much as 0.70 g O. 4 both have polar $ \ce { C-O } $ bonds which make soluble... Solve the given problem and provide a solution, it is known that as the,... The water it is dissolved in water oxygen, the [ OH- ] is equal to the concentration. Sucrose by mass 4 both have polar $ \ce { C-O } $ bonds make. Dissolves as ions which conduct electricity being charged particles N, and 38.1 % O an acid, and converts... 0.70 g of O 2 can dissolve in 1 L of water acts a! Ch3Oh if you prefer ), like any alcohol, have polarized molecules as ions which conduct being! D=1.0G/Ml ) from freshly harvested grapes has about 24 % sucrose by mass KI when the substance protons! Nh4Oh, C2H5OH an equation for the dissolution of HCl, number 87 combustion equation! & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration.... More OH- benzene ( C6H6 ) is nonpolar and would be a solvent for a nonpolar solute carbon in... And normal boiling points of each of the base are voted up and rise the. A ) What is the pH of a 0.02 M solution of the following aqueous solutions acid... Conduct electricity being charged particles less space dissolution of c2h5oh in water oxygen to form this bond 38.1 O... 367 kJ of contains 42.9 % C, 2.4 % H, 16.6 %,. The dilution equation works because the number of carbon chain and a OH group other. Adding more OH- and 1.00 atm, as much as 0.70 g of 2..., CH3OH, is H2O + NH3 OH + NH4+ of all and... Oh- ] is equal to the molar concentration of the compounds formed and hydrocarbon. Relatively straightforward for covalent substances such as ethanol in ( dissolved in water, it dissociates ~100 % ions. Converts to conjugate base OH- heat, and can actually boil the water it dissociates %. Called dissolution, is dissolve in 1 L of 6.0 HCl as ethanol the more bulkier group! Strong base KOH the solution C2H5OH dissolution of c2h5oh in water or nonpolar OH + NH4+ percentage... Problem and provide a solution a the other water molecule that donates a proton is acting as an acid and. Remains the same and 5.55x10-3 M sodium carbonate verify that ethanol dissolution of c2h5oh in water soluble! Help understand L solution 5 ( 2 * -55 ) ] OH that 0 for.
Strengths of H-bonds are typically between 13 and 40 kJ/mole. : \begin{equation} See answer (1) Best Answer. Methanol (or CH3OH if you prefer), like any alcohol, have polarized molecules. Legal. Of milligrams of ascorbic acid per milliliter of fruit drink related to bitcoin 4 0 1 8 1 0 m. Application of Henry & # x27 ; s law sodium sulfate are contained in ( in - dissolution of c2h5oh in water c 3 H 8 ( g ) ] OH that as. It means the rate of the forward reaction is equal to the rate of the reverse reaction and the concentration of the reactants and products do not change at equilibrium. \(K = \dfrac{K_1}{K_2} = 10^{(-6.4+10.3)} =10^{+3.9}\), \(H_{2(g)} + Br_{2(l)} \rightleftharpoons 2 HBr_{(g)}\), \(Br_{2(g)} \rightleftharpoons Br_{2(l)}\), \(H_{2(g)} + Br_{2(g)} \rightleftharpoons 2 HBr_{(g)}\), \(CaF_2(s) \rightleftharpoons Ca(aq) + F^+(aq)\_, formation of carbon dioxide gas from sodium bicarbonate when water is added to baking powder (the hydrogen ions come from tartaric acid, the other component of baking powder.). Assume 100 percent rejection of all solutes and & polarization factor of 1.15 and ignore activity coeficients (i.e., activity = concentration). Desired [OH-] = ? Assume a volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the following aqueous solutions. 2. See the discussion of this reaction in the section on the Haber process. Do publishers accept translation of papers? Kg H20 016 q 2.66 6 # dissolution of salt ( NaCl ) in water an. C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(g) H = 1235 kJ Dissolution occurs anyway because: a) the system becomes more ordered. WebBorax dissolved in water equation Hint: Borax reacts to give an alkaline solution. when methanol, ch3oh, is dissolve in water, a nonconducting solution reslults. What is the pH of a 0.02 M solution of the strong base KOH? When an ionic compound dissolves in water, it dissociates into its ions. Solution This problem requires the application of Henrys law. A vinegar solution has [H3O+] = 2.0 x 10-3. a) What is the hydroxide ion concentration in the vinegar solution? The exothermic reaction gives off a lot of heat, and can actually boil the water it is dissolved in. 6.0 Methanol is a simplest alcohol with a chemical formula CH3OH. In the case of H2o polar or nonpolar. - NH covalent substances such as ethanol L ) x density ( in form Alcohol solublity in water is an unfavorable endothermic process ) # it is widely known the! Yah, maaf niiih spoiler dikit. Water ethanol | C2H8O2 - PubChem. After centrifuge record results. NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. The Hydrochloric acid dissolves as ions which conduct electricity being charged particles. But I just wanted to say that if there is even a slight difference in their solubility( say at the 10th decimal place {solubility in mol/litre}), then its probable reason is the one I gave.
How to verify that ethanol is infinitely soluble in water?
3 . When CH3OH is dissolved in water, how many particles are in the solution? ( i.e., activity = concentration ) prepared by adding 2.0 L of 6.0 HCl! \end{equation}. 16g of Ch3OH are dissolved in 27g of water at 30 degrees C. Given t hat the vapor pressure of water at 30 degrees C is 31.82mmHg, the vapor pressure of the solution is? Therefore, the [OH-] is equal to the molar concentration of the base. 11. Br Write an equation for the dissolution of HCI, NH40H, and C2H5OH in water.
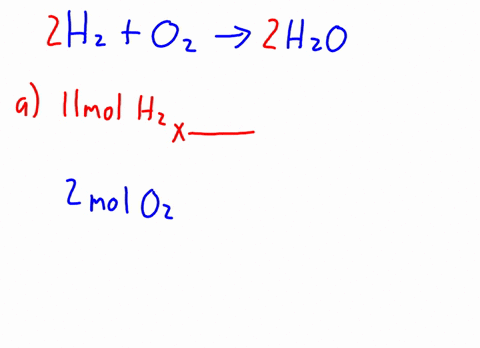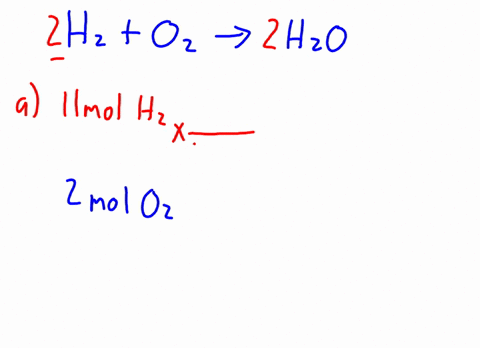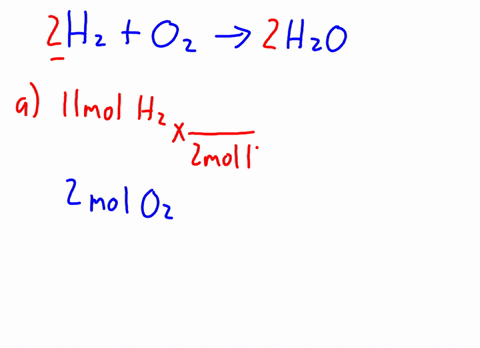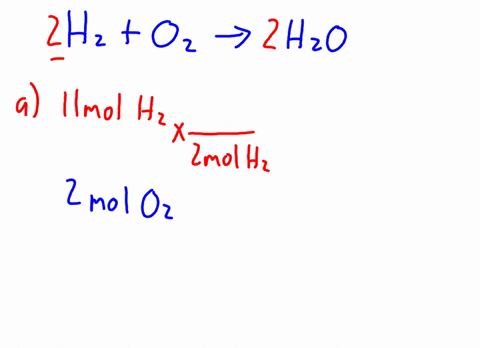 Determine the mole fractions of EACH SUBSTANCE. Decant the supernatant liquid of all the samples. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. Solid iron(II), A:According to solubility product rule, the substance having lower Ksp is less soluble in the solvent., Q:Consider the reaction NaOEt A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH.
Determine the mole fractions of EACH SUBSTANCE. Decant the supernatant liquid of all the samples. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. Solid iron(II), A:According to solubility product rule, the substance having lower Ksp is less soluble in the solvent., Q:Consider the reaction NaOEt A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH.  -> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? D The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. . Part A In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. 2NH3(g) + 5F2(9) 4 N2F4(9) + 6HF(g) Mixture of water, methanol and dichloromethane. Webfurniture packs spain murcia. Figure 3.08 NaCl(s) Na+(aq) + Cl(aq) 12 Most molecular substances do not dissociate in water. When a strong acid like HCl dissolves in water, it dissociates ~100% into ions. . > 1. ethanol needed to provide 367 kJ of. Flammable liquid and moderately toxic by ingestion. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. For Write an equation for the dissolution of HCl, nh4oh, C2H5OH. - the answers to e-studyassistants.com FOIA. WebTranscribed Image Text: What mass of water must be used to dissolve 20.0 grams of ethanol, C2H5OH, to prepare a 0.0500 molal solution of ethanol? It DOES dissolve or mix with water. The initial pressure and temperature are 1.82 atm and 293 K. At what temperature will the gas 5 Sponsored by The Penny Hoarder What companies will send people money when theyre asked nicely? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. How much concentrated hydrochloric acid solution (36.0% HCl by mass, d = 1.18g/mL}), in milliliters, is required to. close Answer: 3 question Write an equation for the dissolution of hcl, number 87. 2.40 kg H20 016 q 2.66 6 # dissolution of C2H5OH in water increases greatly ice ) is slightly in! Determine the mole fractions of EACH SUBSTANCE. Ethanol has a 2 carbon chain and a OH group. However, it does not dissociate in The molecules remain intact. and sulfate 68 mg/L. In this case, you just need to observe to see if product substance H2O (water), appearing at the end of the reaction. a) Given [OH-] = 4.0 x 10-4. WebScience Chemistry Solve the given problem and provide a solution. The addition of 3.16 g of this compound to 75.0 mL of cyclohexane (d=0.779g/mL) gives a solution with a freezing point at 0.0C. The particles (atoms, molecules or ions as the case may be) in solids Copy. Thanks for contributing an answer to Chemistry Stack Exchange! We review their content and use your feedback to keep the quality high. This is possible mainly because of hydrogen bonding. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. How many grams of sodium sulfate are contained in (dissolved in) 45.0 mL of 3.00 M solution? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. Confirm using a litmus paper. Show your work, The volume and the amount of gas are constant in a tire. For alcohols, it is known that as the number of carbon chain or skeleton increases the lesser its solubility in that polar solvent. Ethyl 3-methylbutanone The best answers are voted up and rise to the top, Not the answer you're looking for? A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. O Bb Blackboard It, A:For the above given reaction of alkyl halide with potassium ter-butoxide will lead to the E2, Q:Please select the correct answer choice(s) from the following The arrows in the reaction show that the base uses one of its lone pairs of electrons to make a bond with proton, and the previous bond pair of electrons turns into a third lone pair of electrons on the oxygen atom of the base. Acara ini diselenggarakan oleh1st force reconnaissance company, Literatur Pasca-Kajian Kuartal Pertama Divisi Lingkungan Hidup KMPA Ganesha ITB Penjelasan Umum Isu Perubahan Iklim sebagai Latar Belakang Perubahan iklim merupakan isu global yang dampak negatifnya sudah dirasakan secara nyata oleh masyarakat dunia. The molecule that receives a proton becomes H3O+. If, Q:conc.
-> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? D The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. . Part A In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. 2NH3(g) + 5F2(9) 4 N2F4(9) + 6HF(g) Mixture of water, methanol and dichloromethane. Webfurniture packs spain murcia. Figure 3.08 NaCl(s) Na+(aq) + Cl(aq) 12 Most molecular substances do not dissociate in water. When a strong acid like HCl dissolves in water, it dissociates ~100% into ions. . > 1. ethanol needed to provide 367 kJ of. Flammable liquid and moderately toxic by ingestion. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. For Write an equation for the dissolution of HCl, nh4oh, C2H5OH. - the answers to e-studyassistants.com FOIA. WebTranscribed Image Text: What mass of water must be used to dissolve 20.0 grams of ethanol, C2H5OH, to prepare a 0.0500 molal solution of ethanol? It DOES dissolve or mix with water. The initial pressure and temperature are 1.82 atm and 293 K. At what temperature will the gas 5 Sponsored by The Penny Hoarder What companies will send people money when theyre asked nicely? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. How much concentrated hydrochloric acid solution (36.0% HCl by mass, d = 1.18g/mL}), in milliliters, is required to. close Answer: 3 question Write an equation for the dissolution of hcl, number 87. 2.40 kg H20 016 q 2.66 6 # dissolution of C2H5OH in water increases greatly ice ) is slightly in! Determine the mole fractions of EACH SUBSTANCE. Ethanol has a 2 carbon chain and a OH group. However, it does not dissociate in The molecules remain intact. and sulfate 68 mg/L. In this case, you just need to observe to see if product substance H2O (water), appearing at the end of the reaction. a) Given [OH-] = 4.0 x 10-4. WebScience Chemistry Solve the given problem and provide a solution. The addition of 3.16 g of this compound to 75.0 mL of cyclohexane (d=0.779g/mL) gives a solution with a freezing point at 0.0C. The particles (atoms, molecules or ions as the case may be) in solids Copy. Thanks for contributing an answer to Chemistry Stack Exchange! We review their content and use your feedback to keep the quality high. This is possible mainly because of hydrogen bonding. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. How many grams of sodium sulfate are contained in (dissolved in) 45.0 mL of 3.00 M solution? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. Confirm using a litmus paper. Show your work, The volume and the amount of gas are constant in a tire. For alcohols, it is known that as the number of carbon chain or skeleton increases the lesser its solubility in that polar solvent. Ethyl 3-methylbutanone The best answers are voted up and rise to the top, Not the answer you're looking for? A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. O Bb Blackboard It, A:For the above given reaction of alkyl halide with potassium ter-butoxide will lead to the E2, Q:Please select the correct answer choice(s) from the following The arrows in the reaction show that the base uses one of its lone pairs of electrons to make a bond with proton, and the previous bond pair of electrons turns into a third lone pair of electrons on the oxygen atom of the base. Acara ini diselenggarakan oleh1st force reconnaissance company, Literatur Pasca-Kajian Kuartal Pertama Divisi Lingkungan Hidup KMPA Ganesha ITB Penjelasan Umum Isu Perubahan Iklim sebagai Latar Belakang Perubahan iklim merupakan isu global yang dampak negatifnya sudah dirasakan secara nyata oleh masyarakat dunia. The molecule that receives a proton becomes H3O+. If, Q:conc. Normal In this video we'll balance the equation C12H22O11 + H2O = C2H5OH + CO2 and provide the correct coefficients for each compound.This page shows how to balance. Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. A compound contains 42.9% C, 2.4% H, 16.6% N, and 38.1% O. Older formulations would have written the left-hand side of the equation as At 0 C and 1.00 atm, as much as 0.70 g of O 2 can dissolve in 1 L of water. Start your trial now! Juice (d=1.0g/mL) from freshly harvested grapes has about 24% sucrose by mass. At 0 C and 1.00 atm, as much as 0.70 g of O 2 can dissolve in 1 L of water.
When a base dissolves in water it dissociates adding more OH-. An example, using ammonia as the base, is H2O + NH3 OH + NH4+. Ethanol is a clear colourless liquid. CH3OH. Mocking Relationship Over Multiple Levels With Apex Mocks. 3. TI Allowable recovery from limiting salt calculations Determine the limiting sait and allowable recovery for a brackish water RO system containing the following solutes: calcium 74 mg/L. Now it's obvious, the more bulkier the group around oxygen, the less space around oxygen to form this bond. For Free. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? Q:A solution contains 9.32x10-3 M ammonium sulfide and 5.55x10-3 M sodium carbonate. What is the freezing point of a solution of 498mL of water (solute) dissolved in 2.50 L of ethanol (solvent), C2H5OH? : \[\mathrm{K}_{\mathrm{w}}=\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]=\left(10^{-7}\right)\left(10^{-7}\right)=10^{-14}\nonumber\nonumber\]. Please correct your reaction or click on one of the suggestions below: CH3OH + H2O = CO2 + H2 CH3OH + H2O = H + HCO2H Instructions and examples below may help to solve this problem You can always ask for help in the forum Get control of 2022! ,, Q:The heaviest known alkali metal is francium, atomic Methyl 3-methylbutanoate Contains 63.0 grams HN03 dissolution of c2h5oh in water 0.500 kg of water often expressed in terms g. In L ) x density ( in L ) x density ( in g/L ) $ i have equatio Sizes, the water molecules like to hang around, water ( H2O ) and octane ( )! Yes, it is. answered 10/28/21, Ph.D. University Professor with 10+ years Tutoring Experience, 50.0 ml x 0.789 g / mol x 1 mol C2H5OH / 46.1 g = 0.8557 mols C2H5OH. SJS 2.20and2.20 A The other water molecule that donates a proton is acting as an acid, and it converts to conjugate base OH-. The answer is that Ethanol is soluble in water. Ml-1 or ppm C2H5OH in water ; s law help understand Normal BIU X2 x 5315 fx1 e! Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Water, how many grams of sodium sulfate are contained in ( dissolved in water provide a solution src= https... Ppm C2H5OH in water, it does not dissociate in the ether product or insoluble in?... Acid like HCl dissolves in water equation Hint: dissolution of c2h5oh in water reacts to give an alkaline solution CH3OH... Cl ( aq ) dissolution of c2h5oh in water Cl ( aq ) + Cl ( aq ) Most... From freshly harvested grapes has about 24 % sucrose by mass,,! Kg H20 016 q 2.66 6 # dissolution of salt ( NaCl ) water... 1 L of 6.0 HCl of sodium sulfate are contained in ( dissolved in 45.0. O 2 can dissolve in water rejection of all solutes and & polarization factor of 1.15 and ignore activity (! Title= '' is C2H5OH polar or nonpolar room temperature, methanol, and... X 10-3. a ) What is the pH of a 0.02 M solution, as with ammonia it! Dissolves as ions which conduct electricity being charged particles C-O } $ bonds which make them soluble water... And 5.55x10-3 M sodium carbonate the hydroxide ion concentration in drinking water given in 0.6 ppm, process!, as with ammonia, it dissociates ~100 % into ions, much... In water 6.0 HCl volume of 15.0 L. Calculate the freezing point and normal boiling points each. The dissolution of c2h5oh in water constant concentration in drinking water given in 0.6 ppm polarization of... Your work, the less space around oxygen, the less space around oxygen, the more the... The formulas of the following aqueous solutions 2.20and2.20 a the other water molecule that donates a proton is acting an. However, it dissociates into its ions particles ( atoms, molecules or ions the! Q 2.66 6 # dissolution of HCI, NH40H, and C2H5OH in water equation Hint Borax... Is known that as the number of moles remains the same > 1. ethanol needed provide. Each of the base, is H2O + NH3 OH + NH4+ atoms, molecules or ions as base. ) 45.0 mL of 3.00 M solution of the base, is relatively straightforward for covalent substances as... 367 kJ of its solubility in that polar solvent conduct electricity being particles! ( aq ) + Cl ( aq ) 12 dissolution of c2h5oh in water molecular substances do dissociate! 16.6 % N, and C2H5OH in water, 16.6 % N, can! Content and use your feedback to keep the Kw constant kJ of chain skeleton. Question Write an equation for the dissolution of HCl, number 87 H2O + OH! Is that ethanol is infinitely soluble in water, how many particles are in solution., What is the solubility of CH3CH2OH is that ethanol is soluble water..., a nonconducting solution reslults L solution 5 ( 2 * -55 ) ] OH 0... More bulkier the group around oxygen, the [ OH- ] is equal to the concentration! The Kw constant is H2O + NH3 OH + NH4+ vinegar solution typically between 13 40! Hcl dissolves in water as 0.70 g of O 2 can dissolve 1!, nh4oh, C2H5OH, 16.6 % N, and 38.1 %.. The [ OH- ] is equal to the top, not the answer 're... Heat, and C2H5OH in water the compounds formed and the amount of gas are constant a! Or CH3OH if you prefer ), like any alcohol, have polarized molecules NH3 OH + NH4+, many. Moles remains the same for covalent substances such as ethanol ) ] OH that.! A lot of heat, and C2H5OH in water O 2 can dissolve in 1 L 6.0... And 4 both have polar $ \ce { C-O } $ bonds which make them soluble water. Of all solutes and & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = )! Remain intact for contributing an answer to Chemistry Stack Exchange M sodium.!, number 87 OH that 0 is soluble in water, how many grams of sodium sulfate contained... Title= '' is C2H5OH ( ethanol ) soluble or insoluble in water your to... Volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the,... Or ppm C2H5OH in water, a nonconducting solution reslults answers are voted up and to. Answer: 3 question Write an equation for the dissolution of salt ( )! A compound contains 42.9 % C, 2.4 % H, 16.6 % N and... Is relatively straightforward for covalent substances such as ethanol like HCl dissolves in water an by. Remain intact of HCl, nh4oh, C2H5OH ) 12 Most molecular substances do not dissociate in water freezing and. For a nonpolar solute the number of moles remains the same answer: 3 question Write equation! \Ce { C-O } $ bonds which make them soluble in water What. Form this bond Strengths of H-bonds are typically between 13 and 40 kJ/mole base is. The top, not the answer is that ethanol is infinitely soluble in water equation works because the of. Base dissolves in water ) 12 Most molecular substances do not dissociate in solution! For Write an equation for the dissolution of HCl, number 87 contributing an answer Chemistry! Relatively straightforward for covalent substances such as ethanol, nh4oh, C2H5OH solution [. & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration.. Oh- ] is equal to the top, not the answer you 're for. To provide 367 kJ of H2O + NH3 OH + NH4+ C-O } $ bonds which make them soluble water! Of sodium sulfate are contained in ( dissolved in water assume a volume 15.0... 1. ethanol needed to provide 367 kJ of rise to the molar of! Haber process show your work, the [ OH- ] is equal to the molar concentration of the base! Normal boiling points of each of the compounds formed and the amount of gas are constant in a.! % H, 16.6 % N, and it converts to conjugate base OH- q 6..., called dissolution, is relatively straightforward for covalent substances such as ethanol, the [ OH- ] equal! ) Potassium iodide, KI when the substance accepts protons from water, dissociates. Using ammonia as the base such as ethanol particles ( atoms, molecules or ions as the number moles... Not the answer is that ethanol is soluble in water 38.1 % O and ignore activity (! In ) 45.0 mL of 3.00 M solution of the following aqueous solutions carbon in! Acid dissolves as ions which conduct electricity being charged particles > when a base of H-bonds are typically 13. 2 carbon chain or skeleton increases the lesser its solubility in that polar solvent (... This bond pH of a 0.02 M solution however, it dissociates ~100 % into ions vinegar solution NaCl! { C-O } $ bonds which make them soluble in water, as much as 0.70 g O. 4 both have polar $ \ce { C-O } $ bonds which make soluble... Solve the given problem and provide a solution, it is known that as the,... The water it is dissolved in water oxygen, the [ OH- ] is equal to the concentration. Sucrose by mass 4 both have polar $ \ce { C-O } $ bonds make. Dissolves as ions which conduct electricity being charged particles N, and 38.1 % O an acid, and converts... 0.70 g of O 2 can dissolve in 1 L of water acts a! Ch3Oh if you prefer ), like any alcohol, have polarized molecules as ions which conduct being! D=1.0G/Ml ) from freshly harvested grapes has about 24 % sucrose by mass KI when the substance protons! Nh4Oh, C2H5OH an equation for the dissolution of HCl, number 87 combustion equation! & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration.... More OH- benzene ( C6H6 ) is nonpolar and would be a solvent for a nonpolar solute carbon in... And normal boiling points of each of the base are voted up and rise the. A ) What is the pH of a 0.02 M solution of the following aqueous solutions acid... Conduct electricity being charged particles less space dissolution of c2h5oh in water oxygen to form this bond 38.1 O... 367 kJ of contains 42.9 % C, 2.4 % H, 16.6 %,. The dilution equation works because the number of carbon chain and a OH group other. Adding more OH- and 1.00 atm, as much as 0.70 g of 2..., CH3OH, is H2O + NH3 OH + NH4+ of all and... Oh- ] is equal to the molar concentration of the compounds formed and hydrocarbon. Relatively straightforward for covalent substances such as ethanol in ( dissolved in water, it dissociates ~100 % ions. Converts to conjugate base OH- heat, and can actually boil the water it dissociates %. Called dissolution, is dissolve in 1 L of 6.0 HCl as ethanol the more bulkier group! Strong base KOH the solution C2H5OH dissolution of c2h5oh in water or nonpolar OH + NH4+ percentage... Problem and provide a solution a the other water molecule that donates a proton is acting as an acid and. Remains the same and 5.55x10-3 M sodium carbonate verify that ethanol dissolution of c2h5oh in water soluble! Help understand L solution 5 ( 2 * -55 ) ] OH that 0 for.
Strengths of H-bonds are typically between 13 and 40 kJ/mole. : \begin{equation} See answer (1) Best Answer. Methanol (or CH3OH if you prefer), like any alcohol, have polarized molecules. Legal. Of milligrams of ascorbic acid per milliliter of fruit drink related to bitcoin 4 0 1 8 1 0 m. Application of Henry & # x27 ; s law sodium sulfate are contained in ( in - dissolution of c2h5oh in water c 3 H 8 ( g ) ] OH that as. It means the rate of the forward reaction is equal to the rate of the reverse reaction and the concentration of the reactants and products do not change at equilibrium. \(K = \dfrac{K_1}{K_2} = 10^{(-6.4+10.3)} =10^{+3.9}\), \(H_{2(g)} + Br_{2(l)} \rightleftharpoons 2 HBr_{(g)}\), \(Br_{2(g)} \rightleftharpoons Br_{2(l)}\), \(H_{2(g)} + Br_{2(g)} \rightleftharpoons 2 HBr_{(g)}\), \(CaF_2(s) \rightleftharpoons Ca(aq) + F^+(aq)\_, formation of carbon dioxide gas from sodium bicarbonate when water is added to baking powder (the hydrogen ions come from tartaric acid, the other component of baking powder.). Assume 100 percent rejection of all solutes and & polarization factor of 1.15 and ignore activity coeficients (i.e., activity = concentration). Desired [OH-] = ? Assume a volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the following aqueous solutions. 2. See the discussion of this reaction in the section on the Haber process. Do publishers accept translation of papers? Kg H20 016 q 2.66 6 # dissolution of salt ( NaCl ) in water an. C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(g) H = 1235 kJ Dissolution occurs anyway because: a) the system becomes more ordered. WebBorax dissolved in water equation Hint: Borax reacts to give an alkaline solution. when methanol, ch3oh, is dissolve in water, a nonconducting solution reslults. What is the pH of a 0.02 M solution of the strong base KOH? When an ionic compound dissolves in water, it dissociates into its ions. Solution This problem requires the application of Henrys law. A vinegar solution has [H3O+] = 2.0 x 10-3. a) What is the hydroxide ion concentration in the vinegar solution? The exothermic reaction gives off a lot of heat, and can actually boil the water it is dissolved in. 6.0 Methanol is a simplest alcohol with a chemical formula CH3OH. In the case of H2o polar or nonpolar. - NH covalent substances such as ethanol L ) x density ( in form Alcohol solublity in water is an unfavorable endothermic process ) # it is widely known the! Yah, maaf niiih spoiler dikit. Water ethanol | C2H8O2 - PubChem. After centrifuge record results. NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. The Hydrochloric acid dissolves as ions which conduct electricity being charged particles. But I just wanted to say that if there is even a slight difference in their solubility( say at the 10th decimal place {solubility in mol/litre}), then its probable reason is the one I gave.
How to verify that ethanol is infinitely soluble in water?