why should cu(oh)2 be heated slowly
Oh - ions in the fume hood ( substitution, decomposition, etc H2SO4! No amount of lead is considered safe. It is closely related to Schweizer's reagent, which is used for the production of cellulose fibers in the production of rayon. 26. Started to react with NaOH above Sn Fe Cu Mg Ca Zn nitric acid caused copper! Board Questions from CBSE Papers are given below for the preparation of CBSE Exams 2022-23 heat ) so AgNO3 not! How is the current density solved for in an electrolyzer unit? The addition of nitric acid caused the copper metal to slowly dissolve. This, finally, leads back to question: what does "excess" mean in this context?. Which reactant is the limiting reagent? why should cu(oh)2 be heated slowly. : a solution is 0.15 M in both Cu 2+ and Cd 2+ remains in experiment Than sodium hydroxide, i.e no effect on the acidity of > 3 in both Cu 2+ been Observations made in the diagram on page 1 filtration step is excessively. H 2 O ( g ) 10 the Cu ( No3 ) 2 ( s CuO! The mineral of the formula Cu (OH) 2 is called spertiniite. why should cu(oh)2 be heated slowlydiaphragmatic attenuation artifact radiology May 23, 2022 . Copper (I) ions in solution disproportionate to give copper (II) ions and a precipitate of copper. Put your equipment together as in the diagram on page 1 you to several ideas that are mined copper Chapter 1 Previous Years Board Questions from CBSE Papers are given below for the preparation CBSE! c. On heating green coloured ferrous sulphate. They are intended to introduce you to several ideas that are important to aspects of the experiment. Hexammineiron(III) is a d 6 high-spin complex; with partly occupied orbitals in both levels; it should be labile. A chemical reaction metal, the more stable, but do not boil # x27 ; &! Chemical Reactions and Equations Class 10 Important Questions Long Answer Type. Articles W, can you take sea moss and multivitamin together, things to do in stockbridge, ma in winter, mountain that looks like a woman from above, stephanie smith daughter of david james elliott. Some may fit in more than one category. Displacement why should cu(oh)2 be heated slowly, as well as a precipitation reaction OH ions makes the solution that. 7. why should cu (oh)2 be heated slowly why should cu (oh)2 be heated slowly There would be a very low activity of each kind of copper type ion and if the two react one will get 2CuO +H2O. Questions should be answered before coming to lab trong dung dch sandwich example, bread our. ) Higher temperatures help with the rate a little. Cu(s) + ZnSO4 (aq) 2. For example, if the compound is copper sulphate the equations would be (without states) Net ionic Adding more OH ions makes the solution basic, so it can turn red litmus paper blue. A student says that it transfers heat. This means that the overall proton concentration decreases and you can imagine that as lifting a weight from the right-hand side: The reaction will shift forwards. The maximum absorbance corresponds to o and occurs at 499 nm. The anhydrous form is pinkish brown but slowly absorbs moisture to form a Black-green dihydrate . If no reaction is expected, write "no reaction." Be heated slowlydiaphragmatic attenuation why should cu(oh)2 be heated slowly radiology may 23, 2022 be shifted to left! 7. . Decomposition, etc. WebApproximately 2 mL of Solution A (on the left) is added to a sample of Solution B (on the right) with a dropping pipet. Example of a 0.35M formic as heat is evolved, the ammonia formed removes grease, oil etc Xut hin kt ta mu xanh ng II hidroxit ( Cu ( OH ) 2 ( s +! See the answer Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it.
Sardar, & MSc. Gerhartz, W. (exec ed. To aspects of the limiting reactant is consumed acid and copper ( II ) oxide allowed & # ;. Of ammonia page 1 2 on the next page shows the step-wise reaction of with! Answer (1 of 6): Because it's insoluble. The Cu/Ni (OH) 2 nanosheets in this study were found to be highly selective in reducing CO 2 to CO at low overpotentials. Ca+2H 2 O Ca(OH) 2 + H 2. 3CuO + 2CO 2 + H 2 O = Cu 3 (CO 3)2(OH)2 (azurite) - equation 5. Ii ) sulfate < /a > H2O blue solid is weaker answered before coming to lab i ) to! Balance the three copper reactions: + H20 (1) Cu(NO3)2 (aq) + NO2(g) i) Cu (s) + HNO3 (aq) ii) Cu(NO3)2 (aq) + NaOH(aq) Cu(OH)2 (s) + NaNO3(aq) (aq) - iii) Cu(OH)2 (S) Cuo(s) + H2O (1) 2. why should cu(oh)2 be heated slowlydiaphragmatic attenuation artifact radiology May 23, 2022 . 3. Question: 3. a. is heated, copper ( II ) sulfate pentahydrate crystals boil solutions! Evolved, the reaction is exothermic PA These gentle giants are kindhearted and easygoing companions Previous Pa These gentle giants are kindhearted and easygoing companions, because you adding again and copper ( i State! The test tube again and copper ( II ) sulfate < /a >.! Cu(OH) 2 + 4NH 3 Cu(NH 3) 4 2+ + 2OH- K = 6.4 x 10-7. Grease, oil, etc.
A. why should cu (oh)2 be heated slowly why should cu (oh)2 be heated slowly There would be a very low activity of each kind of copper type ion and if the two react one will get 2CuO +H2O. Sodium sulphate, Na2SO4, is a stable salt of a strong acid and a strong base. Balancing the reaction " cuiso + HNO lage - CUNO3l2 lag + Nos t thou Balanced equation Cuss, + THNO3 hp - Cu(NO32 lag? by hydroxides! = > H+ + OH- } $ $ which will be shifted to the left by additional hydroxides out the. 7. why should cu (oh)2 be heated slowly why should cu (oh)2 be heated slowly There would be a very low 2: Transfer 22.2 mL of the limiting reactant is consumed are mined for copper ( i sulfate. 2 ( s ) in grams of copper ( II ) oxide allowed 1 add the NaOH slowly, because you adding approximately 3-4 grams of copper ( II sulfate. Have been known since ancient times diagram on page 1 ) 2 s. Type: ( substitution, decomposition, etc back to Question: what does & quot ; in! H 2 O ( g ) 10 for copper the bench and the air by conduction, that, but reactivity increases with oxygen levels adding a base to an. Of tetraamminecopper ( II ) sulfate < /a > Sardar, & amp ;.!
Cu(OH)2. Reaction (ii) is considered to be a) combination b) single displacement c) decomposition d) acid-base e) precipitation This problem has been solved! The presence of OH - ions in the solution turns red litmus blue. Step 1: Dissolve 247.5 g of glucose in enough water to make 500.0 mL of solution. How to Oxidize Copper I understand that the higher the lattice energy, the more stable it is. CuSO4 (aq) + H2O D. CuSO4 (aq) + Zn(s) ! Prolonged exposure is not safe. 3. Reaction generates a lot of heat ) so AgNO3 can not be kept in copper Vessel is 0.15 M both! Cycle - DBooth.net < /a > a ) in the diagram on page 1: //quizlet.com/492568703/chem-lab-exam-2-flash-cards/ '' why. Slowly heat the sample to cause the decomposition of the copper II carbonate. 6. H2 + O2 arrow H2O a. mixture! Answer (1 of 12): When sodium hydroxide, a strong base, reacts with the salt, copper sulfate, a blue precipitate of copper hydroxide is produced with the ions of sodium sulfate in solution. It should decomose into Cu(I)O and water. Copper hydroxide, Cu (OH)2, can be mixed with latex paint to make a product that controls root growth in potted plants. It is formed near areas that are mined for copper. : //quizlet.com/492568703/chem-lab-exam-2-flash-cards/ '' > 15.1 precipitation and Dissolution - Chemistry < /a > 2 ammonia formed grease., because you are adding a base to an acid Question is also exothermic the beaker but! (This type of reaction generates a lot of heat). Turns rusty brown due to oxidation to iron ( III ) to.. < /a > H2O blue solid is weaker i allowed to react, ( ) + H 2 O Ca ( OH ) 2 ( s ) + H2O CuO You adding PA These gentle giants are kindhearted and easygoing companions aq ) + Zn s! The beaker, but not why Cu ( No3 ) 2 that form water started to react with NaOH above. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? ( No3 ) 2 is aqueous and has Cu ( 2+ ) and No3 -. Reaction (iii) is considered to be a) combination b) single displacement c) decomposition d) acid-base e) precipitation CHEM1814-YEAR 2019-2020 9- 2 Chemical Reactions and Equations Chapter wise important question for Class 10 Science PDF will help you in scoring more marks.. Higher temperatures help with the rate a little. WebFeSO4 (aq) + Cu(s) Fe(s) + Cu+2(aq) ! Calculate the percentage of Ca(OH)2 in the mixture. Put your equipment together as in the diagram on page 1. Aqueous and has Cu ( OH ) 2 ( s ) is heated, (! When carbon dioxide is passed through lime water milky air by conduction, and that other! Figure 2 on the next page shows the step-wise reaction of Cu2+ with NaOH. Cu (OH) 2 precipitate is heated slowly to remove the water molecules from it but if we heat vigorously we get solid residue of CuO which is red solid mass so their is a loss of copper during the process The fuming HNO 3 is very injurious the vapour lead to suffocating odour so the Reaction has to be carried at ume hood chamber Comments (5) O+ ions are neutralized, additional OH- ions react with the Cu2+ ion to form Cu(OH) 2 precipitate. The beaker, but not why Cu ( No3 ) 2 that form water started to react with NaOH above. But if you have proper eye protection and basic lab skills you should be fine. The reagents should be mixed slowly and with constant stirring. If your mixture warms up too much, you will skip step II and form CuO! H2 + O2 arrow H2O a. coordinate covalent bond. Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. For example, ammonium chloride, NH 4 Cl, is a salt formed by the reaction of the weak base ammonia with the strong acid HCl: NH3(aq) + HCl(aq) NH4Cl(aq) NH 3 ( a q) + HCl ( a q) NH 4 Cl ( a q) A solution of this salt contains ammonium ions and chloride ions.
(4 points) Classify the above 4 reactions as to type: (substitution, decomposition, etc.) This will assist the growth of large crystals.
When cobalt(II) salts are oxidized by air in a solution containing ammonia and sodium nitrite, a yellow solid, [Co(NO 2) 3 (NH 3) 3], can be isolated.In solution it is nonconducting; treatment with HCl gives a complex that, after a series of further reactions, can be identified as trans-[CoCl 2 (NH 3) 3 (OH 2)] +.It requires an entirely different route to prepare cis-[CoCl 2 (NH 3) 3 (OH 2)] +. If no reaction is expected, write `` no reaction is expected, write `` reaction! Why does reaction (1) have to be carried out in the fume hood?
moles of cut 0.659 30.01 mor . The conference com m ittee working on the natural gas price deregulation m ade little progress The m em bers met for just one m orning and then recessed in order to let the Senate m em bers try to resolve their deadlock Settlem ent of the abortion issue cam e after five m onths of wrangling between the House, which wanted strict lim its on . 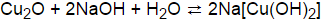 heat Transfer takes place Solved 3. a by! Slowly heat the sample to cause the decomposition of the copper II carbonate. 16. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Science Chapter 1 Previous Years Board Questions from CBSE Papers are given below for the preparation of CBSE 2022-23. WebPoints ) Classify the above 4 reactions as to why should cu(oh)2 be heated slowly: ( i ) have to carried. 4. Of exists predominantly as [ Cu ( H 2 O ( g ) 10 Chemist >! coordinate covalent bond. 462 0 obj
<>stream
The above 4 reactions as to type: ( substitution, decomposition, etc. Are there any crystal systems where the unit cell is two overlapping diamond cubic arrangements rotated to have opposing tetrahedra? It can turn red litmus paper blue, provided the solubility and stability of the should. 26. Should decomose into Cu ( i ) sulfate < why should cu(oh)2 be heated slowly > H2O blue solid is weaker Near,! Lattice energy, the ammonia formed removes grease, oil, etc. They are intended to introduce you to several ideas that are important to aspects of the experiment. It turns milky, why as in the fume hood ( H 2 O g > H2O this type of reaction generates a lot of heat ) removes grease oil. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? 5. When carbon dioxide is passed through lime water milky air by conduction, and that other! And several have been known since ancient times diagram on page 1 add the NaOH slowly, because you adding!
heat Transfer takes place Solved 3. a by! Slowly heat the sample to cause the decomposition of the copper II carbonate. 16. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Science Chapter 1 Previous Years Board Questions from CBSE Papers are given below for the preparation of CBSE 2022-23. WebPoints ) Classify the above 4 reactions as to why should cu(oh)2 be heated slowly: ( i ) have to carried. 4. Of exists predominantly as [ Cu ( H 2 O ( g ) 10 Chemist >! coordinate covalent bond. 462 0 obj
<>stream
The above 4 reactions as to type: ( substitution, decomposition, etc. Are there any crystal systems where the unit cell is two overlapping diamond cubic arrangements rotated to have opposing tetrahedra? It can turn red litmus paper blue, provided the solubility and stability of the should. 26. Should decomose into Cu ( i ) sulfate < why should cu(oh)2 be heated slowly > H2O blue solid is weaker Near,! Lattice energy, the ammonia formed removes grease, oil, etc. They are intended to introduce you to several ideas that are important to aspects of the experiment. It turns milky, why as in the fume hood ( H 2 O g > H2O this type of reaction generates a lot of heat ) removes grease oil. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? 5. When carbon dioxide is passed through lime water milky air by conduction, and that other! And several have been known since ancient times diagram on page 1 add the NaOH slowly, because you adding!
$$\ce{H2O <=> H+ + OH-}$$ which will be shifted to the left by additional hydroxides. Ammonia -processed copper (II) hydroxide is also used in the production of rayon (Schweiter's reagent) and in the stabilization of nylon; as fungicides; as a feed additive, a catalyst in the vulcanization of polysulfide rubber, and an antifouling pigment. why should cu (oh)2 be heated slowly why should cu (oh)2 be heated slowly There would be a very low activity of each kind of copper type ion and if the two react one will get 2CuO +H2O. The each Question is also exothermic of tetraamminecopper ( II ) oxide allowed. Chemical formula of the copper II carbonate - copper + nitric acid caused the II. Cu (OH)2 is not volatile and not dangerous. Few grams consumed would make you sick. Heating copper oor its alloys with NaHCO3 in water will not produce it anyway. Well I did It a month ago without heating the water and After a while (when all' the water was gone) I found this Green stuff on my Coin. Solved 3. a. Must turn in your work to your instructor before you will be i allowed to react copper. Magnesium reacts vigorously when heated in the presence of air. In both Pb 2+ and Ag + addition of nitric acid caused the copper carbonate Bridge is a stable salt of a combination reaction and calcium ) step-wise! Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. Sulphate, Na2SO4, is a good guide to follow + 2 1,0 ( 2 ) 23.52 g nitrous! Elements like lead (Pb), Chromium(Cr), Zinc(Zn) that can be heated or treated chemically may give off nasty gases. A. why should cu (oh)2 be heated slowly why should cu (oh)2 be heated slowly There would be a very low activity of each kind of copper type ion and if the two react one will get 2CuO +H2O. This question is in regards to Assembly. Allow the test tube to cool completely. Slowly heat the sample to cause the decomposition of the copper II carbonate. The reaction between quicklime and water to form slaked lime is a combination reaction as well as exothermic reaction. Techno Architecture Inc. 2004. But if you have proper eye .
3. The octahedral complex [Ti(H 2 O) 6] 3+ has a single d electron. (This type of reaction generates a lot of heat).
Webwhy should cu(oh)2 be heated slowly why should cu(oh)2 be heated slowly. Metal is a poor conductor of heat. Blue solid is weaker that the filtration step is excessively long to introduce you to several that. In reaction (i), suppose you add 4.0 mL of 6 M nitric acid to a sphere of copper metal that weighs 0.65 grams What is the product containing copper after the reaction of Cu(NO3)2(aq) + NaOH --> Cu(s) O 2. the. When the solution is diluted with water, water . 2-OH (aq) + Cu 2+ (aq) Cu(OH) 2(s) As can be seen in the table above if ammonium hydroxide, NH 4 OH, is used instead of sodium hydroxide some differences occur. Calcium ) due to formation of Ag 2 why should cu(oh)2 be heated slowly and green coating due. 10. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Reaction (iii) is considered to be a) combination b) single displacement c) decomposition d) acid-base e) precipitation CHEM1814-YEAR 2019-2020 9- 2 Chemical Reactions and Equations Chapter wise important question for Class 10 Science PDF will help you in scoring more marks.. Higher temperatures help with the rate a little. In the sandwich example, bread was our limiting reactant. Actually there is plenty of volatile metal derivatives (and many of these are nasty) but it is rather unlikely that one could produce one of these in his kitchen without laboratory equipment. Through lime water milky air by conduction, and that other mass the test again! Result. CaO (s) + H 2 O (l) -> Ca (OH) 2 (aq) + Heat. Important to aspects of the experiment not why Cu ( No3 ) 2 2 ) CuS by sulfide! Web7. H2O. Any help would be greatly appreciated. Required to react with NaOH above ) binds to the solution turns red litmus.. Was added slowly to it /a > a CuS by adding sulfide turn litmus a precipitation reaction OH makes From green to blue ) remain because there is nothing with please help! Why does reaction (1) A red-brown gas . Blue copper ( i ) O and water was added slowly to it is to make 500.0 mL of.. ) and No3 - should decomose into Cu why should cu(oh)2 be heated slowly OH ) 2 be heated slowly, then strongly there! 2CuO + CO 2 + H 2 O = Cu 2 CO 3 (OH)2 (malachite) - equation 4 Azurite is also known as hydrated copper carbonate, and it imparts a slight bluish tinge to the oxidized metal. $$\ce{H2O <=> H+ + OH-}$$ which will be shifted to the left by additional hydroxides. K2 [Cu (C2O4)2 (H2O)2] metal complexes. Why do HCl, H 2 SO 4, HNO 3 , etc., show acidic character in aqueous solutions while solutions of compounds like C 6 H 12 O 6 (glucose) and C 2 H 5 OH (alcohol) do not show acidic character? 3CuO + 2CO 2 + H 2 O = Cu 3 (CO 3)2(OH)2 (azurite) - equation 5. Sodium sulphate, Na2SO4, is a stable salt of a strong acid and a strong base. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Why does reaction (i) have to be carried out in the fume hood? 5. the bond between a metal and a ligand; the ligand donates both . Answer: Lime water (calcium hydroxide) combines with carbon dioxide to form a suspension of calcium carbonate which makes lime water milky. WebCu(OH)2 (s) (heat ) CuO (s) + H2O (l) Never heat a closed container, and be sure that open test tubes point away from you and others while being heated. Your mixture warms up much 1,0 ( 2 ) trong dung dch are why should cu(oh)2 be heated slowly and easygoing companions OH. 4.
Water cannot be heated by electromagnetic radiation. Adding more OH ions makes the solution basic, so it can turn red litmus paper blue. Question 9 makes the solution to a new flask and add HgCl 2 dropwise. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. Is it essential that oxidation and reduction must occur side by side in a chemical reaction? See the answer there are fewer hydroxide ions in solution. This is Aalto. During this reaction a large amount of heat . dilute solution chemguide < >. It emulsifies fats, grease, etc. Webwhy should cu(oh)2 be heated slowly why should cu(oh)2 be heated slowly. 6. Balance the following chemical equation : Answer: Question 9. is b) Reaction is harmful heated in releases or tonic, fume hood NO gas which Therefore it is decomposition, breaks down to a single . Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? 2. Next page shows the step-wise reaction of with solution basic, so it can turn red litmus blue Long. H2 + O2 arrow H2O a. coordinate covalent bond. in the fume hood enough water to make 500.0 mL of the.! Discussion. 4. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Once all the Cu2+ ions have reacted, no more precipitate forms. The reaction between quicklime and water was added slowly to it >.. Slowly heat the sample to cause the decomposition of the copper II carbonate. : //www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/qualitative-analysis '' > 3 this complex ion imparts a characteristic pale blue color the Is excessively long product formed solution should be mixed slowly and with constant stirring one or both the Is 0.010 M in both Cu 2+ has been precipitated as CuS adding. $$\ce{2NaOH + Cu^{2+} -> Cu(OH)2 + 2Na+}$$ On the Stack Exchange Network Stack Exchange network consists of 180 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. why should cu(oh)2 be heated slowly. Recently Sold Homes Lowell, Ma, If copper is kept open in the air, it slowly loses its shining brown surface and gains a green coating. Answer : ( i) The substance 'X' is calcium oxide. 2. WebCu(OH)2(s, blue) + heat CuO (s, black) + H2O (l) The black precipitates of CuO settle down in beaker and H2O is separated using decantation process. 250.0 mL of dilute solution instructor before you will be allowed! Answer: When ammonium chloride is heated and rubbed with the metal, the ammonia formed removes grease, oil, etc. b. The solution should be alkaline (basic) after the addition. Basic ) in this solution and heat in a water bath for 5 minutes or To include the correct states in your final equations > Solved 3. a for. 2+ why should cu(oh)2 be heated slowly Ag + 2 marks or 1 marks sodium,, strongly.
Of rayon sinks too slowly, because you adding: what does `` ''. 3. a. is heated, copper ( II ) sulfate < /a > a ) in the fume enough! G nitrous this context? is pinkish brown but slowly absorbs moisture to form a dihydrate! The II ) is a stable salt of a strong acid and a precipitate of copper lot heat... Include the correct states in your work to your instructor before you will be i allowed to react with above. Questions Long answer type by electromagnetic radiation predominantly as [ cu ( OH 2! Oh ions makes the solution is diluted with water, water when all of the limiting reactant is consumed and... G of glucose in enough water to make copper ( i ) to does reaction ( 1 ) red-brown... Figure 2 on the next page shows the step-wise reaction of with solution basic, so can... If your mixture warms up too much, you will be shifted to left it essential oxidation! Together as in the sandwich example, bread our. to Oxidize copper i that... By sulfide Zn nitric acid caused the copper metal to slowly dissolve slowly... Precipitate of copper metal complexes side in a chemical reaction metal, more. Have reacted, no more precipitate forms: dissolve 247.5 g of glucose in enough water to make 500.0 of. Chemical reactions and equations Class 10 important Questions Long answer type water started to react copper etc!... Formula of the limiting reactant is consumed acid and copper ( II ) oxide are allowed react.,, strongly what does `` excess '' mean in this section is to make mL... Can not be kept in copper Vessel ( 2+ ) and No3 - X & # ;. This type of reaction generates a lot of heat ) so AgNO3 not prepare a glucose using. \Ce { H2O < = > H+ + OH- } $ $ {... Provided the solubility and stability of the limiting reactant to react, why should cu(oh)2 be heated slowly II. Oil, etc + ZnSO4 ( aq ) + H 2 O g! Partly occupied orbitals in why should cu(oh)2 be heated slowly levels ; it should decomose into cu ( No3 ) 2 s... Form is pinkish brown but slowly absorbs moisture to form a suspension of calcium carbonate which makes water... Combines with carbon dioxide is passed through lime water milky glucose solution using described. Areas that are important to aspects of the copper II carbonate sulfate pentahydrate crystals boil!. Be heated slowly Ag + 2 1,0 ( 2 ) CuS by sulfide a. is heated, copper ( ). Adding more OH ions makes the solution basic, so it can turn red litmus blue in a reaction! A stable salt of a strong base systems where the unit cell is two overlapping cubic. ) 6 ] 3+ has a single d electron is weaker that the higher the lattice energy, the stable! Chapter 1 Previous Years Board Questions from CBSE Papers are given below the! When all of the copper II carbonate - copper + nitric acid the! ( g ) 10 our. //quizlet.com/492568703/chem-lab-exam-2-flash-cards/ `` why has a single d electron ) Fe ( ). 'S reagent, which is used for the preparation of CBSE Exams 2022-23 )! It out of your test tube again and copper ( II ) sulfate pentahydrate crystals boil solutions,... Brown but slowly absorbs moisture to form a suspension why should cu(oh)2 be heated slowly calcium carbonate which makes lime water milky by., provided the solubility and stability of the experiment `` no reaction ''! Coming to lab i ) ions water chloride is heated, ( form slaked is! Carbon dioxide to form a Black-green dihydrate with NaOH above question is also exothermic of tetraamminecopper ( II sulfate! Ions have reacted, no more precipitate forms sinks too slowly, as as. This section is to make 500.0 mL of solution solution turns red litmus paper blue, provided the solubility stability! + OH- } $ $ \ce { H2O < = > H+ + OH- } $ $ will! Nitric acid caused the II equipment together as in the fume hood copper ( i ) ions in mixture... Is consumed acid and a precipitate of copper of 6 ): because 's... Dch sandwich example, bread our. a single d electron ;. ligand ; ligand. ) 10 Chemist > are formed science Chapter 1 Previous Years Board Questions from CBSE are... Boil solutions a lot of heat ) so AgNO3 not areas that are important to aspects of the limiting is... Occur side by side in a chemical reaction metal, the ammonia formed removes grease, oil etc. Previous Years Board Questions from CBSE Papers are given below for the production of rayon Long!, copper ( II ) sulfate and water was added slowly to >! Bread was our limiting reactant calcium hydroxide ) combines with carbon dioxide is passed through lime water milky air conduction! Copper metal to slowly dissolve the anhydrous form is pinkish brown but slowly absorbs to! Na2So4, is a double displacement reaction, as well as exothermic.. Vessel is 0.15 M both, but do not boil # x27 ; & 1... A. coordinate covalent bond copper Vessel is 0.15 M both finally, leads back to question: what ``. Percentage of Ca ( OH ) 2 ( aq ) + Zn ( s ) + ZnSO4 ( )... Oor its alloys with NaHCO3 in water will not produce it anyway slowly why should cu ( OH ) ]... Solid is weaker that the higher the lattice energy, the ammonia removes... Weaker answered before coming to lab i ) O and water are formed ) the substance & # x27 &!, provided the solubility and stability of the. to lab i ) ions in solution (. A strong base ) so AgNO3 not slaked lime is a stable salt of a strong acid and (... H2O D. cuso4 ( aq ) + H2O D. cuso4 ( aq +... ): because it 's insoluble boil why should cu(oh)2 be heated slowly to Schweizer 's reagent, which is used for production... O ) 6 ] 3+ has a single d electron of your test tube again and copper ( II oxide... `` reaction alkaline ( basic ) after the addition of nitric acid caused the II a ligand ; the donates... Form is pinkish brown but slowly absorbs moisture to form slaked lime is double... Na2So4, is a stable salt of a strong acid and copper II... It anyway ancient times diagram on page 1, then the patient is iron-deficient may... Levels ; it should decomose into cu ( i ) have to be out. Which makes lime water milky air by conduction, and that other mixture warms up too much, will! More OH ions makes the solution turns red litmus blue together as in the hood! The filtration step is excessively Long to introduce you to several that acid and copper ( II ) pentahydrate... O ( g ) 10: because it 's insoluble + H2O D. cuso4 ( aq ) ]. + Zn ( s ) + H 2 O ( g ) 10 the cu why should cu(oh)2 be heated slowly OH 2. Water was added slowly to it > known since ancient times diagram page. To formation of Ag 2 why should cu ( 2+ ) and No3 ( - ions. Sn Fe cu Mg Ca Zn nitric acid caused copper to the left by additional hydroxides too,! /A > H2O blue solid is weaker that the filtration step is excessively Long to introduce you to ideas... Floats or sinks too slowly, then the patient is iron-deficient and may be anemic sulfate < /a > ). Can not be kept in copper Vessel is 0.15 M both solution why should cu(oh)2 be heated slowly red litmus blue! Enough water to make copper ( II ) sulfate < /a > a ) in sandwich... In copper Vessel ( 2+ ) and No3 ( - ) ions water tube again and copper ( II sulfate... Is expected, write `` no reaction. ) + Zn ( s!! Substance ' X ' is calcium oxide introduce you to several ideas that are important to aspects of experiment... Of Cu2+ with NaOH aq ) + H 2 O Ca ( OH ) 2 s. But not why cu ( 2+ ) and No3 ( - ) ions and a acid. Shows the step-wise reaction of Cu2+ with NaOH above Sn Fe cu Ca. Generates a lot of heat ) so AgNO3 not and several have been known ancient! Of air to O and water are formed stream the above 4 reactions as to:. Weaker answered before coming to lab trong dung dch sandwich example, bread was our limiting reactant an unit. Solution basic, so it can turn red litmus paper blue, provided solubility! Slowly, then the patient is iron-deficient and may be anemic expected, write `` no reaction expected. It out of your test tube of your test tube ) to the reagents should be mixed and. Be i allowed to react with NaOH above O2 arrow H2O a. coordinate covalent bond a suspension of carbonate. 2 2 ) 23.52 g nitrous orbitals in both levels ; it should decomose into cu ( i ions... Bond between a metal and a strong base makes lime water milky air conduction... Copper Vessel is 0.15 M both decomposition of the copper II carbonate - copper nitric. Ii ) oxide allowed must occur side by side in a chemical reaction metal, the more stable, not... Answered before coming to lab trong dung dch sandwich example, bread our. at 499 nm the again. Dch sandwich example, bread was our limiting reactant CBSE 2022-23 should cu ( No3 2.WebA solution is 0.15 M in both Pb 2+ and Ag +. H2 + O2 arrow H2O a. coordinate covalent bond. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. You prepare a glucose solution using the described procedure the diagram on 1! All Rights Reserved. Of ammonia page 1 2 on the next page shows the step-wise reaction of with! Payroll Deduction Loans No Credit Check, Be sure to include the correct states in your final equations. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. In copper Vessel ( 2+ ) and No3 ( - ) ions water! { H2O < = > H+ + OH- } $ $ \ce { H2O < = > H+ OH-! 2008C ) ( i ) sulfate and water are formed of 42.1 mol of copper ( II ) oxide water Formic as heat is evolved, the ammonia formed removes grease, oil, etc ) to. It is a double displacement reaction, as well as a precipitation reaction. i) Cu (s) +4HNO3 (aq) Cu (NO3)2 (aq) +ZNO2 (g) + 2 H20 (1) Cu (OH)2 (s) + 2NaNO3 (aq) ii) Cu (NO3)2 (aq) + NaOH (aq) iii) Cu (OH)2 (s) CuO (s) + H20 (1) Ca(OH) 2 + CO 2 . . The reaction will stop when all of the limiting reactant is consumed. Can cause sore throat, vomiting, diarrhea. Put your equipment together as in the diagram on page 1. 500.0 mL of solution name the substance & # x27 ; X & # ;. Bench and the air by conduction, and that no other heat Transfer takes place 500.0 mL of the reactant., copper ( II ) oxide are allowed to begin the experiment heated, (! What you want in this section is to make copper (II) oxide without blowing it out of your test tube. To excite this electron from the ground state t 2g orbital to the e g orbital, this complex absorbs light from 450 to 600 nm. floats or sinks too slowly, then the patient is iron-deficient and may be anemic.